⇧ [VIDÉO] You might also like this partner content
A trial involving nearly 500 adults shows that electrical stimulation of the vagus nerve (VNS) significantly improves the symptoms of severe depression resistant to conventional treatments. Before these trials, patients had tried more than ten treatments without seeing any improvement. This research aims to provide the evidence necessary for SNV to be covered by health insurance in the United States.
Major depressive disorder (MDD) is a chronic and disabling condition that interferes with daily activities and places people at increased risk of suicide. If standard antidepressants allow remission in more than 50% of patients, around 30% remain unresponsive to four successive lines of treatment.
Initially developed to treat drug-resistant epilepsy, vagus nerve stimulation (VNS) has, for more than two decades, been proposed as a long-term treatment for chronic and resistant depression (at least four failed treatments) in adults. In 2005, the US Food and Drug Administration (FDA) approved this approach for the management of resistant depression in the United States.
However, the accessibility of this therapy remains restricted, in particular due to the cost of the device and the surgery necessary for its implantation. In addition, clinical trials aimed at demonstrating its effectiveness have so far delivered contrasting results. In particular, in 2007, the Centers for Medicare and Medicaid Services (CMS) ruled that the available evidence was not sufficient to justify medical coverage.
The RECOVER study, led by Washington University in Saint-Louis, aims to reassess the effectiveness of SNV in the treatment of severe resistant depression and bipolar disorder. It also aims to collect data likely to convince the CMS. The results, published in two articles in the journal Brain Stimulationcould influence the decisions of private insurers, often aligned with those of the CMS.
Measurable progress during the last months of treatment
The VNS device, similar to a pacemaker, is surgically implanted under the skin in the chest. It emits electrical impulses via a wire connected to the left vagus nerve, which transmits stimuli to brain regions involved in mood regulation.
The trial recruited 493 adults with severe resistant depression across 84 sites. Three-quarters of these patients, severely depressed, were unable to work. On average, they had tried 13 treatments without success. All participants were already receiving treatment and were encouraged to continue it alongside the trial, while continuing to consult their doctors.
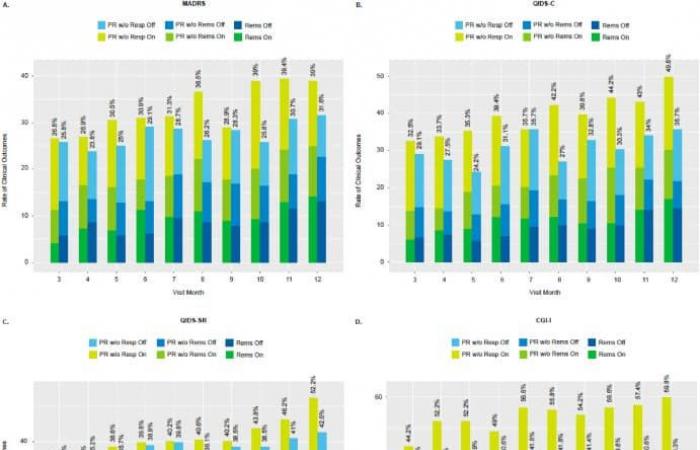
All participants were implanted, but only 249 devices were activated during the 12-month randomized control phase. The first two months were dedicated to optimizing the stimulation parameters for each patient. From the third month onwards, a monthly symptom assessment was carried out. Four reference tools were used, including two by blinded external clinicians, another by an on-site professional, and a final one by patient self-assessment. They also assessed their quality of life and their ability to carry out daily tasks every three months using validated tools.
Stacked bars of response and remission rates at monthly intervals starting 3 months after baseline in imputed data. © Conway et al.
Overall, participants whose devices were activated saw a notable reduction in their symptoms, accompanied by an improvement in their quality of life and ability to perform daily tasks. However, this progress was only observed from the last three months of treatment. This latency, the researchers explain, is explained by the progressive nature of the effects of SNV.
See also


A complete remission rate still limited
However, the complete remission rate remained low and did not differ significantly between the two groups. “ What matters here is that patients themselves report an improvement in their quality of life », Underlines Charles Conway, lead author of the studies, in a press release. “ These are patients who have failed a considerable number of treatments, including aggressive approaches such as electroconvulsive therapy. And they don’t just say ‘I’m a little better.’ They report seeing significant improvements in their ability to function and lead their lives ».
At the end of the trial period, the devices of participants in the control group were activated. No notable differences were observed between groups, although control patients reported unexpected improvement in their symptoms. This improvement could be attributed to a placebo effect, with participants being informed that their device would be activated later.
Despite this, “what is encouraging about VNS, and confirmed by other studies, is that when a patient responds to treatment, the effects are generally long-lasting,” adds Conway. Participants will be followed for four additional years to assess the sustainability of the effects and identify the patient profiles most receptive to the therapy.