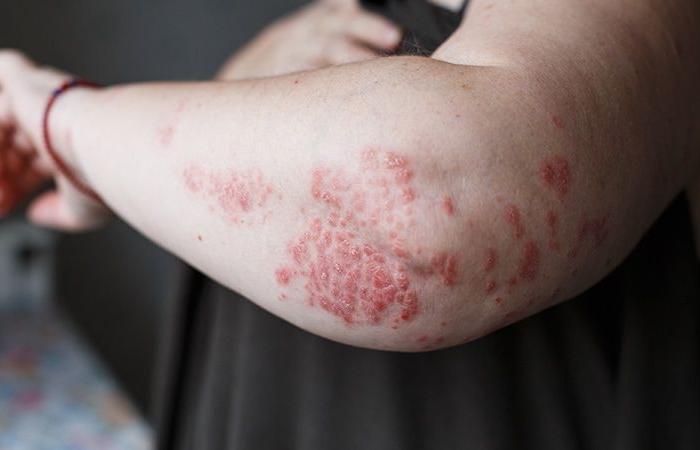
In approximately 20% of patients suffering from psoriasis, the disease occupies at least 10% of the body’s skin surface, which may warrant systemic treatment. Injectable biotherapies blocking interleukin 17 or interleukin 23 are very effective in this indication. But for many, having an oral alternative would be welcome.
A promising target for new oral drugs for psoriasis and other inflammatory dermatoses is tyrosine kinase 2 (Tyk2), a member of the JAK family involved in several intracellular signaling pathways including interleukins 23 and 12 and interleukins 23 and 12. type 1 interferon which contributes to certain autoimmune diseases (psoriasis, lupus, IBD, etc.).
Deucravacitinib (Sotyktu®) an oral allosteric Tyk2 inhibitor is now available for treatment (in 3thline) of moderate to severe psoriasis. Another oral allosteric inhibitor of Tyk2, zasocitinib, is also expected in this indication: it has the same mechanism of action as deucravacitinb but it would have greater selectivity for the “pseudokinase” domain (JH2 for AS homologs 2) from Tyk2.
A phase 2b trial (randomized, double-blind controlled vs. placebo) evaluated the efficacy, safety and tolerability of zasocitinib at different dosages in the treatment of moderate to severe psoriasis. It took place from August 11, 2021 to September 12, 2022 in 47 centers in the United States and 8 in Canada. The inclusion criteria were: age between 18 and 70 years, PASI score (psoriasis area and severity index) of at least 12, a PGA score (Physician’s Global Assesment) of 3 or more and a body surface covered by psoriasis plaques of more than 10%.
A third of patients “cured” at a dose of 30 mg per day
A total of 259 patients, including 82 women, with a mean age of 47 years (SD 13) were randomized to receive zasocitinib at a dose of 2, 5, 15 or 30 mg per day or a placebo for 12 weeks.
At week 12, a PASI 75, i.e. an improvement of at least 75% of the initial score, was obtained in 9 (18%), 23 (44%), 36 (68%) and 35 (67 %) patients respectively on 2, 5, 15 or 30 mg of zasocitinib and in 3 patients on placebo.
The results for PASI 90 and PGA of 0 and 1 were consistent with those observed for the primary endpoint (PASI 75 at 12 weeks): PASI 90 was achieved by 45% of patients on 15 mg and 46% of those on 30 mg; the PGA score of 0 or 1 concerned 49% of patients on 15 mg and 52% on 30 mg.
A dose response relationship was observed with an increase in the number of responses in parallel with the increase in dosage, with 17 patients (33%) on the highest dose of 30 mg of zasocitinib benefiting from a disappearance of the lesions (PASI 100). Furthermore, improvement is evident from the first 4 weeks of treatment.
Adverse effects during treatment were noted in 23 patients on placebo and 28 to 31 on different doses of zasocitinib: these were mainly acneiform dermatitis, diarrhea and Covid 19. There was no change in parameters. hematological or liver function.
As in studies with deucravacitinib, there was no MACE (Major Adverse Cardiovascular Events) or thromboembolic events such as can be deplored with anti-JAKs.
These results suggest that zasocitinib could claim a place of choice alongside biotherapies and other oral treatments subject to confirmation of its effectiveness and tolerance by larger phase 3 studies.