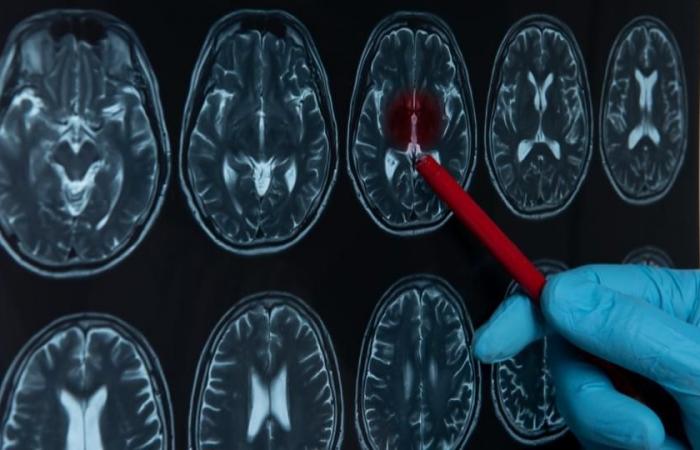
The drug Androcur, notably prescribed against endometriosis or as a means of contraception, is linked to an increased risk of meningioma. An association of victims filed a complaint against X in Paris, this Tuesday, November 5.
A “bankruptcy” of the actors in charge of security around Androcur. This is what Amavea, an association representing victims of this drug, denounces in a complaint against X filed in Paris, this Tuesday, November 5. This anti-hormonal product has, for years, been prescribed against acne, hair loss but also as a means of contraception or as a solution to endometriosis. Originally, the drug was designed to combat hirsutism, a disorder of hair growth, AFP reported.
Androcur is nevertheless responsible for many potentially serious side effects, and would increase the risk of developing a meningioma, a brain tumor. The complaint filed highlights five criminal offenses, according to information from our colleagues in Le Monde.
In detail, the association denounces the administration of a harmful substance, involuntary harm to the integrity of the person, endangering others, failure to report adverse effects and aggravated deception.
tumor cases declared from 1998
“It is now obvious that the actors in charge of the safety of Androcur – Health Agency, laboratories, doctors – have failed in the management of the side effects of this drug”, deplore Me Charles Joseph-Oudin and Emmanuelle Mignaton, president of Amavaea, in a press release.
“Amavea, representative of thousands of victims, wants an investigation to be carried out to determine the negligence committed and establish the responsibility of the actors involved,” they continued, requesting the appointment of an investigating judge.
According to the complaint, “starting in 1998, cases of meningiomas were regularly reported to laboratories marketing Androcur.”
A RISK INCREASED BY TWENTY
As early as 2007, health professionals had already warned about the risks of meningioma linked to this drug sold by Bayer. In 2018, several epidemiological studies established that at high doses, the risk is multiplied by 7 after six months of treatment. After five years of treatment, the risk increases twentyfold.
For the association’s lawyers, “this surricide, identified by the laboratory in 2004, was then recognized by the firm and the ANSM (Medicine Safety Agency) between 2008 and 2009. However, no information has been communicated to prescribing health professionals or patients before 2019. Or years of silence according to the lawyers.
![Cette pathologie gynécologique concerne une femme sur dix en âge de procréer. [©Adobe]](https://euro.dayfr.com/content/uploads/2024/11/08/f9d8d5994c.jpg)
![Cette pathologie gynécologique concerne une femme sur dix en âge de procréer. [©Adobe]](https://locals.dayfr.cam/wp-content/uploads/2024/11/1731086649_262_what-is-this-drug-suspected-of-causing-brain-tumors.jpg)
It was only in 2019 that the Health Authority and the Laboratory “put in place a risk management plan which included, among other things, the dissemination of targeted information to patients taking Androcur”, they said. also criticized.
women still under treatment
Contacted by AFP, the ANSM “did not wish to make a comment “in this context””.
Androcur prescriptions fell by almost 90% between January 2018 and December 2023, according to the ASNM. At the end of November 2023, fewer than 10,000 patients were still treated with Androcur, compared to around 90,000 under treatment at the end of 2017.
Between 2009 and 2018, more than 2,500 women were operated on for a meningioma, formed after taking a progestin medication. These tumors, which affect the membranes surrounding the brain, are benign, but can still cause significant neurological disabilities.