Japanese researchers have taken an important step in the field of renewable energy. A bioinspired hydrogel could indeed transform the way we produce hydrogen, using sunlight.
Hydrogen, a clean and promising fuel, is at the heart of green energy research. However, its production via photosynthesis artificial was hitherto hampered by limited effectiveness. Japanese scientists have managed to get around this obstacle by creating a hydrogel capable of breaking down water into hydrogen and oxygen using only sunlight.
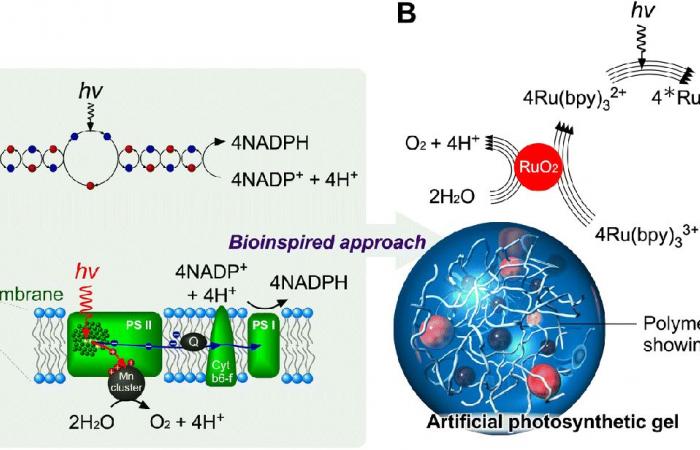
This material, developed by researchers at the Japan Institute of Advanced Science and Technology (JAIST) and the University of Tokyo, incorporates a structure polymer which allows optimal electron transfer. This mechanism is essential for breaking down water molecules into gases. By simplifying this reaction, the hydrogel becomes much more effective than its predecessors.
The major problem with existing artificial photosynthesis systems was the agglomeration of molecules, a phenomenon which slowed down the transfer of electrons. The researchers therefore developed a three-dimensional architecture, aiming to avoid this agglutination. This structure allows for more efficient dissociation of water molecules, thereby increasing hydrogen production.
The hydrogel uses ruthenium complexes and platinum nanoparticles, arranged in a precise organization. This not only guarantees the absence of agglomeration, but also optimizes the transfer of electrons. The result: much higher energy efficiency than previous systems.
(A) Mechanism of natural photosynthesis.
(B) Design of artificial photosynthetic gels.
The production of hydrogen, obtained solely by the action of light on water, offers great potential in the energy transition. The researchers highlight the importance of this innovation for the industrial and transport sectors, where hydrogen could soon replace fossil fuels.
One of the unique characteristics of this hydrogel is the careful structuring of the molecules. The researchers succeeded in creating an environment where the transfer of electrons occurs smoothly, without unwanted side reactions. This progress could have a major impact on the efficiency of clean energy technologies.
However, scientists insist that further developments are needed before large-scale industrialization. The next step will be to optimize the stability of the hydrogel and perfect its production methods. This innovative system created by researchers could well redefine the way we produce hydrogen. But there are still technical challenges to overcome before seeing it deployed industrially.
Researchers are already working to integrate new components into hydrogels to further maximize their performance. Their goal: to transform this discovery into a viable and sustainable solution on a large scale.
What is artificial photosynthesis and how does it work?
Artificial photosynthesis is a process that mimics the natural photosynthesis of plants. It uses sunlight to trigger chemical reactions that generate energy, often in the form of hydrogen, a clean energy source. Unlike natural photosynthesis, which transforms carbon dioxide and water into glucose, the artificial version mainly aims to separate water molecules into hydrogen and oxygen, using materials synthetics.
Artificial photosynthesis systems are often based on materials capable of absorbing light and transferring electrons through a molecular network. These electrons are then used to split water molecules. The main challenge lies in organizing the molecules to ensure that this transfer occurs smoothly, without aggregation that could slow or stop the process.
Materials used for this technology often include hydrogels or bioinspired polymers. These structures, carefully arranged, make it possible to maintain optimal electron transfer and avoid losses in efficiency. By adding metal complexes or nanoparticles, such as ruthenium or platinum, researchers are able to make these systems more efficient, thus increasing hydrogen production.
The ultimate goal of artificial photosynthesis is to produce hydrogen from water and sunlight, without the need for external energy input. This advance could become an alternative to current hydrogen production methods, which are often expensive and energy-intensive. If these systems become more efficient and industrial, hydrogen could play a key role in the energy transition.