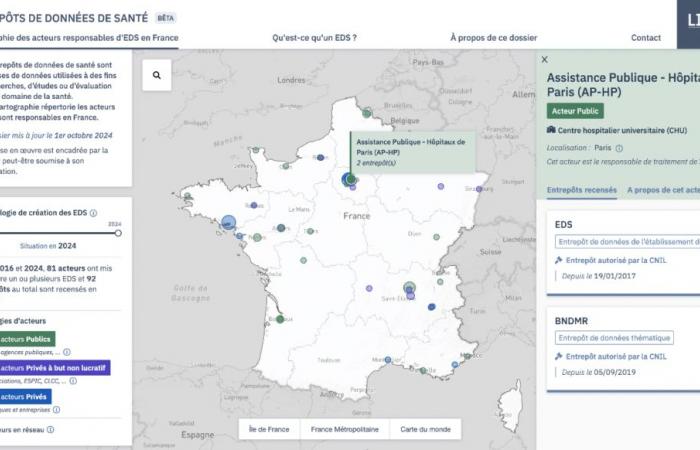
The CNIL Digital Innovation Laboratory (LINC) publishes a map of health data warehouses in France. This tool aims to document the initiatives of different actors who constitute these databases, particularly in the context of research.
What is a health data warehouse (EDS)?
EDS are databases created for a long period and intended to be reused mainly for steering purposes (management, control and administration of the activity) and research, studies, evaluations in the field of health. They can be set up by both public (such as a public healthcare establishment) and private (such as a data broker or a startup) actors, subject to respecting the applicable legal framework.
To find out more about EDS, see the publication “Health data processing: how to distinguish between a warehouse and research and what consequences? » on the CNIL website.
Why is LINC publishing this map?
The CNIL has a role as regulator of personal data in general, and health data in particular. Thus, it supports, authorizes (in certain hypotheses) and controls the implementation of these health data warehouses.
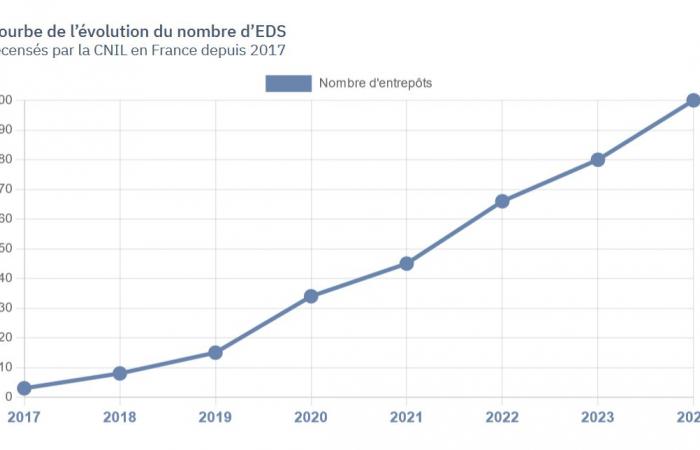
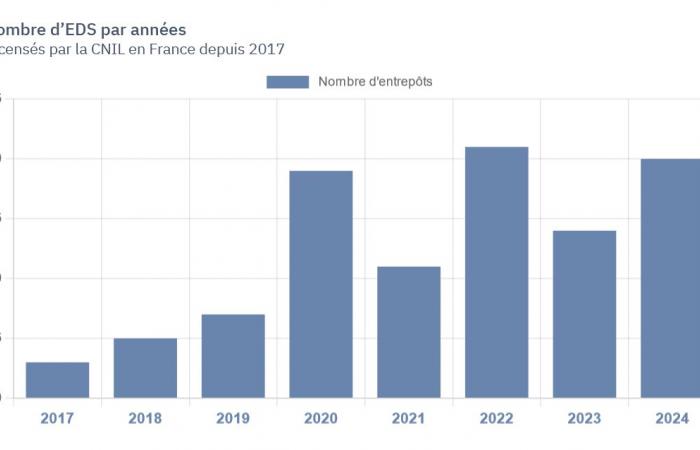
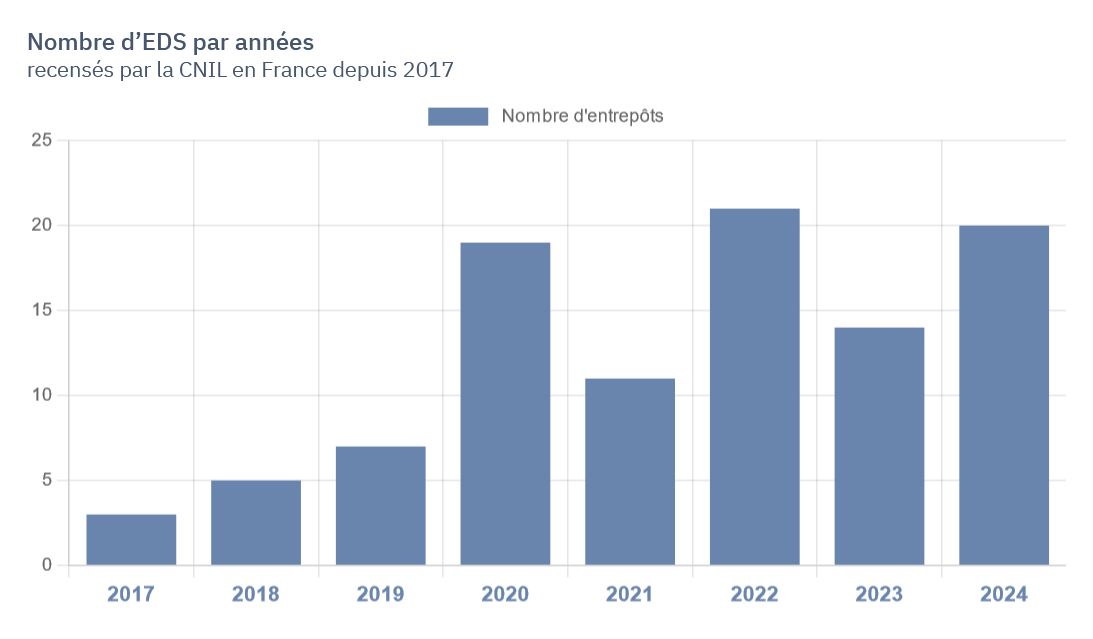
Faced with the proliferation of the latter and the organizations wishing to establish them, it appeared particularly useful to create a tool allowing both to understand the dynamics at work and to improve the transparency of the use of health data in the research framework.
What data is presented in this map?
This mapping also makes it possible to “materialize” the existence of health data warehouses and to remind people (concerned or not) that their data is used in the context of research, management or evaluation of the activity. Symmetrically, project leaders can use this tool to explore current research and find projects that may be relevant to their own research..
This mapping provides access to files on establishments including:
the name and a description of the actor managing the warehouse; the status of the actor (public, private or private non-profit); its geographical location; the name and a description of the warehouse(s) managed by this actor; the date of the warehouse authorization or declaration of conformity (if applicable).For more information on how mapping works, see the dedicated LINC article.
France