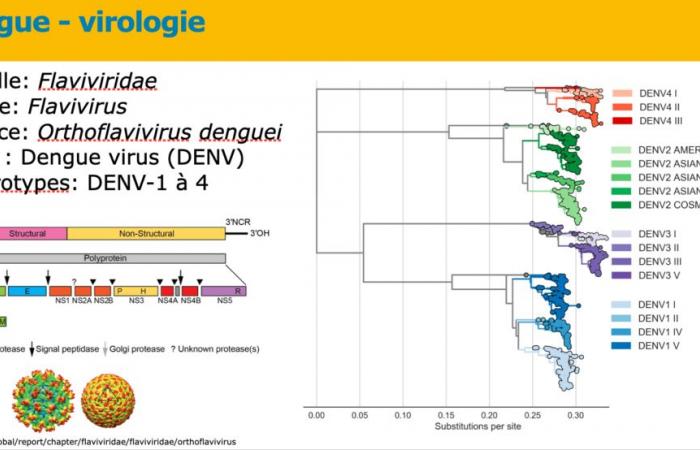
It was one of the highlights of the 25e national infectious disease days (JNI) which took place in Deauville from June 12 to 14, 2024: the Takeda laboratory symposium on dengue at the heart of the fight against this “threat without borders», which followed an oral communications session entirely dedicated to dengue. The presentations notably addressed the risks in France a few weeks before the Olympic Games, whether the cases are imported or indigenous.
The main subjects discussed were the increase in cases of dengue fever in mainland France, the cessation of the marketing of the vaccine available during the communication by Professor André Cabié from the Martinique University Hospital, the announcement of a new promising vaccine against dengue fever. and vector control in a perspective One Health. Also addressed, the more specific question: the risks presented by arboviruses Arboviruses are viral diseases caused by arboviruses transmitted obligatorily by an arthropod vector (mosquito, biting midge, tick) to vertebrate hosts (mammals, birds), hence their name adapted from English: ARthropod-BOrne virus. in the event of pregnancy, beyond the known case of Zika. A vintage of the JNI with, therefore, dengue fever on all levels.
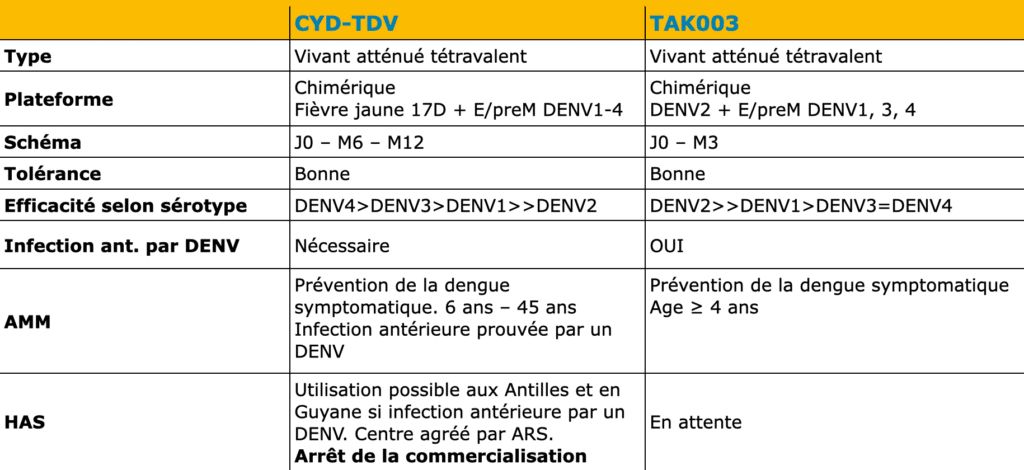
Today, the TAK-003 vaccine (Qdenga®) is now the only dengue vaccine with marketing authorization in Europe. In France, we are waiting for both the recommendations of the technical committee on vaccinations (CTV) and the High Authority of Health (HAS) for this medicine, and of course, to know the setting of its price.
The previous vaccine not recommended in France
In January 2019, a dengue vaccination strategy was defined for the Dengvaxia® vaccine (CYD-TDV). We remember the controversy and legal debate between the Philippine government and the vaccine manufacturer. The latter being accused by the Philippine authorities of having led to hundreds of child deaths as part of one of the largest vaccination operations against dengue fever. Eight hundred thousand children were vaccinated during a campaign where the limitations of the vaccine in terms of serotype and case of serology Study of sera to determine the presence of antibodies directed against antigens. negative, fears and controversies, as presented in this publication by Yu et al., “Fear, mistrust, and vaccine hesitancy: Narratives of the dengue vaccine controversy in the Philippines”.
It was then found that vaccinated HIV-negative participants had twice the risk of severe dengue fever and hospitalization than unvaccinated participants. Vaccination leads to the production of facilitating antibodies which would favor the occurrence of serious form even more than a first infection known to be a risk factor for serious form amplified by comorbidities [âge, obésité, bronchopneumopathie chronique obstructive (BPCO), diabète, etc.].
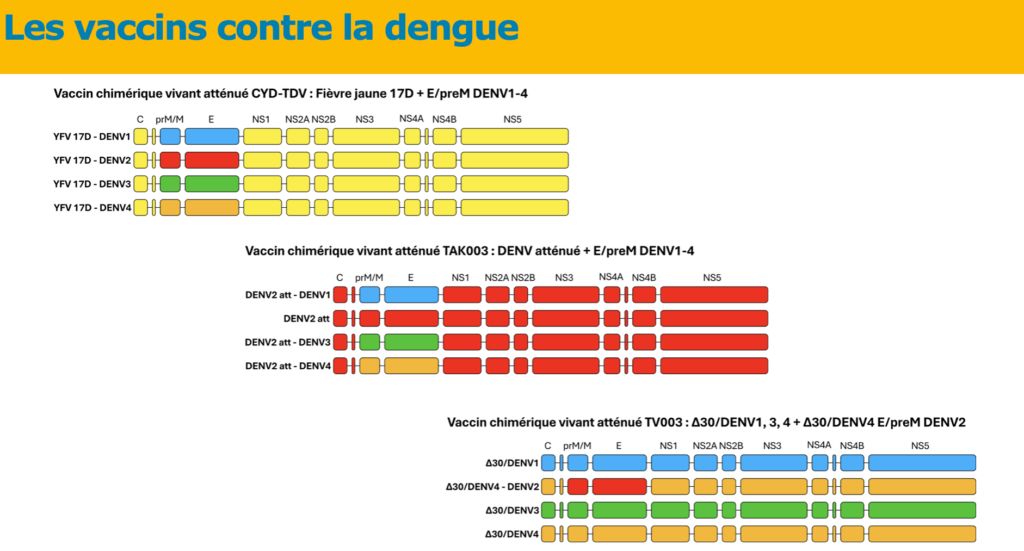
Remember that on December 12, 2018, Dengvaxia® obtained a AMM Marketing Authorization. Administrative procedure which authorizes a pharmaceutical laboratory to market a molecule. European Union restricted to people aged 9 to 45 years with a history of biologically confirmed dengue virus infection, which has significantly limited its use. In 2019, the HAS did not recommend the use of Dengvaxia® on Reunion Island and Mayotte despite the endemic outbreak. These recommendations have not been modified by the new HAS opinion dated June 30, 2022.
Towards a new French vaccination strategy
Following the marketing authorization of Takeda’s vaccine by the European Commission on December 5, 2022, the Directorate General of Health (DGS) contacted the High Authority for Health (HAS) on April 3, 2023 so that it revises its strategy in the face of dengue to include the evaluation of Qdenga®. The DGS wants the evaluation of the Qdenga® vaccine to be carried out based on age, immune status and comorbidities and for the pending recommendations to cover France and overseas departments and regions.
Note that TAK-003 was prequalified by the World Health Organization (WHO) on May 10, 2024, the second vaccine against dengue fever to be prequalified by the WHO. WHO prequalification is an important step in expanding global access to vaccines, as it enables their purchase by organizations across the United Nations system, including UNICEF and the Pan American Health Organization.
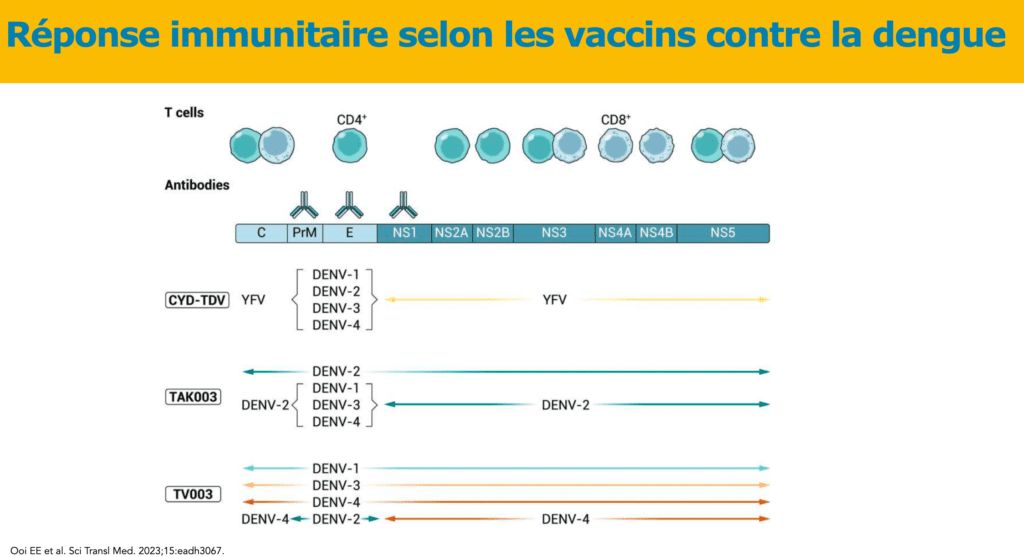
The JNI were an opportunity to recall the typical roadmap for a dengue vaccine: demonstrate cross-protection against the 4 types of virus, reduce the risk inherent in this arbovirus Arboviruses are viral diseases caused by arboviruses transmitted obligatorily by an arthropod vector (mosquito, biting midge, tick) to vertebrate hosts (mammals, birds), hence their name adapted from English: ARthropod-BOrne virus. viral occurrence of facilitating antibodies having a deleterious effect – especially after a first infection with one of the dengue viruses -, circumvent the absence of a satisfactory animal model and the difficulty of having correlates of protection in terms of rate of neutralizing antibodies.
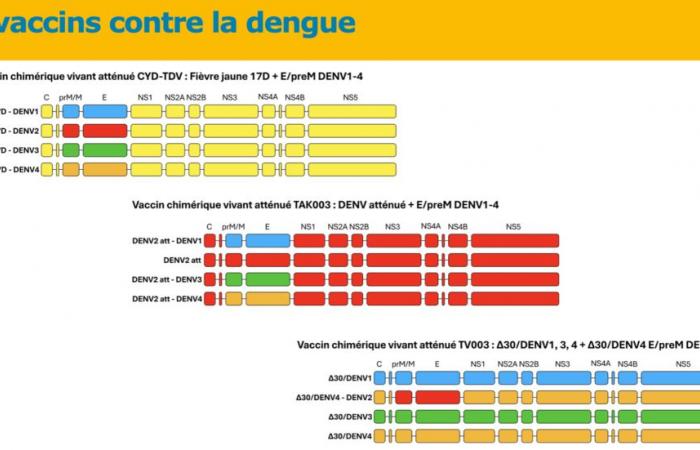
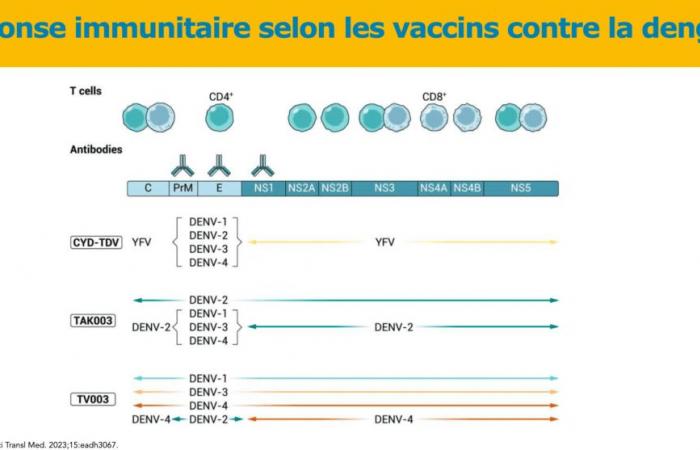
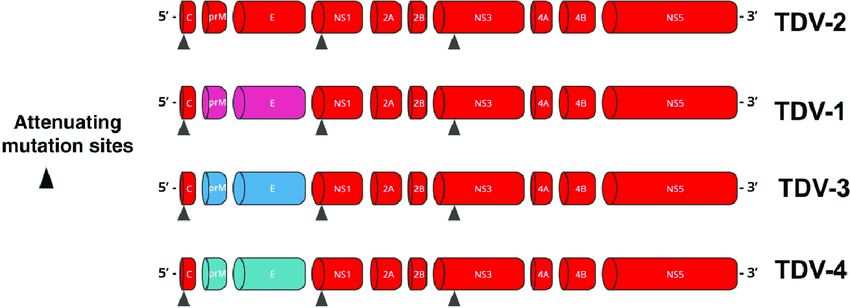
Theoretically, three vaccines have been developed: Sanofi’s CYD-TDV, which is currently withdrawn, Takeda’s TAK-003, and another live attenuated chimeric vaccine, TV003, which is currently in development.
For now, it is this second vaccine that is attracting all eyes. It is a live, tetravalent, chimeric vaccine which, unlike Sanofi’s Dengvaxia® vaccine, uses an attenuated dengue serotype 2 virus (and not the attenuated yellow fever virus) as a basis. The pivotal trial which allowed this marketing authorization began in 2016, with 20,071 children aged 14 to 16 years in a randomized trial with control by placebo Inert substance, without pharmacological activity, having the same appearance as the product with which we wish to compare it. (Editor’s note: nothing to do with the alternative rock group formed in 1994 in London by Brian Molko and Stefan Olsdal.) in 8 countries in Asia and America with high endemic activity. The effectiveness in preventing symptomatic dengue 18 months after the two vaccination injections was overall 80.2% (73.3% – 85.3%), in people seronegative for dengue, the Efficacy was 66.2% and it varies depending on the serotype, ranging from 95% for DENV-2 to 48.9% for DENV-3. Five years after the first injection, this overall effectiveness was still 61.2% (56% – 65.8%). The vaccine effectiveness with respect to the risk of hospitalization is 90.4% 18 months after the second dose No serious adverse effects have been reported in the various publications except for 16 cases of anaphylaxis described in Brazil, including 3 anaphylactic shocks with a low frequency estimated at 0.8 per 100,000 doses.
The WHO has recommended TAK-003 for use in children aged 6 to 16 years in areas with high dengue transmissions. The vaccine must be administered 1 to 2 years before the age corresponding to the peak incident hospitalizations. Knowing that catch-up vaccination can be considered for other age groups, particularly 6-16 year olds and people with comorbidities. Finally, and this is a major subject, but which is not found in all the recommendations, pre-vaccination serological screening, which was a major obstacle for the previous vaccine, is not recommended in the European Marketing Authorization. can specify, as Christophe RAPP (American Hospital) did, that these “high dengue transmission zones” are defined by a seroprevalence at the age of 9 greater than 60% and a peak in dengue hospitalizations before the age of 16.
Need clarification
Furthermore, the Pan American Health Organization (PAHO) aligned itself with WHO recommendations and asked the Takeda laboratory to carry out phase 4 studies to provide answers to the uncertainties about the effectiveness of the vaccine against DENV-3 and DENV-4 in dengue seronegative individuals.
In people who live in countries where dengue is not endemic and who have already been infected with any of the 4 serotypes of the virus following travel, vaccination with TAK-003 may be useful. to prevent a second infection. Protection begins 14 days after the first dose.
If we compare the Belgian, Swiss, German and Spanish recommendations, we see different positions regarding the fact of having already contracted dengue or not to recommend this vaccination. In Belgium and Switzerland, for example, having had dengue fever is part of the indication criteria, whereas in Germany, this vaccination is independent of dengue serological status. We impatiently await clarification from the French authorities on this point.
Unanswered questions
After the disillusionment with the first dengue vaccine, developed from an attenuated strain of the yellow fever virus, and the controversy surrounding child deaths in the Philippines, a number of questions remain.
In particular the question on French territory of vaccine hesitancy, extremely present in the Antilles and Guyana, regions particularly affected by the current dengue epidemic. This hesitation in the face of vaccination had also increased during the coronavirus crisis. Covid Coronavirus disease, sometimes referred to as coronavirus disease, is an illness caused by a coronavirus (CoV). The term may refer to the following diseases: severe acute respiratory syndrome (SARS) caused by the SARS-CoV virus, Middle East respiratory syndrome (MERS) caused by the MERS-CoV virus, coronavirus disease 2019 ( Covid-19) caused by the SARS-CoV-2 virus. No data is currently available on the acceptability, in the Antilles and Guyana, of this type of vaccine and its methods of administration. Same question on the negative impact that the controversy in the Philippines could have had on Dengvaxia®, which had seen a decline in other vaccinations: vaccination against measles will have seen its vaccination coverage drop from 80 to 50%.
It remains that dengue fever constitutes a major public health danger which justifies any vaccine research: the number of cases in the world is estimated to be 100 to 400 million each year and the population living in countries where the disease is 3.8 billion. endemic (Asia, Africa, Americas). Knowing that the year 2023 is the year which saw the highest number of cases of dengue fever ever reported before with 2,300 deaths reported by the World Health Organization and for France, the double record for both dengue indigenous and imported dengue fever which are confirmed for the first months of 2024. In the specific context of France, the question of the acceptance of such a vaccine, after the above-mentioned controversies and the climate of vaccine hesitancy particularly in the Antilles and Guyana, remains a huge question mark.