Unprecedented. This is how the results of the first two PURPOSE studies are qualified, which evaluate the effectiveness and safety of lenacapavir in PrEP Pre-Exposure Prophylaxis. PrEP is a strategy that allows an HIV-negative person exposed to HIV to eliminate the risk of infection, by taking, continuously or “on demand”, anti-retroviral treatment based on Truvada®. injectable: 100% protection against transmission of HIV Human immunodeficiency virus. In English: HIV (Human Immunodeficiency Virus). Isolated in 1983 at the Pasteur Institute in Paris; recent discovery (2008) rewarded with the Nobel Prize in Medicine awarded to Luc Montagnier and Françoise Barré-Sinoussi. among cisgender women, and 96% among men who have sex with men (MSM) and transgender women. As the first phase III trial in the history of HIV prevention to show no infections, PURPOSE 1 (conducted in cisgender women in South Africa and Uganda: link https://vih.org/vih- and-sexual-health/20240704/la-prep-injectable-twice-a-year-the-encouraging-results-of-letude-purpose-1/) has revived the hope of finding a tool that would make it possible to end the transmission of HIV. Currently, the promise of achieving this goal, set for 2030 by the WHO and UNAIDS, continues to recede.
We remember the chairwoman of IAS 2024 in Munich, Sharon Lewin emphasizing the unprecedented results of Pupose I: “Zero! I repeat: zero infections!” While recalling the role of the people concerned in these results: “The PURPOSE clinical trial was only possible thanks to the commitment of thousands of women across Africa at risk of HIV infection who participated in it. . These are the same communities that must have access to it first.”
The impact on prevention
Can this new type of PrEP have a significant impact on HIV prevention globally? Today, the use of oral PrEP remains suboptimal in certain key populations, or even non-existent in others. In sub-Saharan Africa in particular, women, who are most affected by the epidemic, have limited recourse to it. In Northern countries, men who have sex with men (MSM) but who do not identify with the gay community are often less inclined to use PrEP than their gay or bisexual peers. Finally, transgender people’s access to PrEP is often complicated by specific issues of discrimination and precariousness, leading to problems of compliance with oral intake – perhaps as many early cessations or abandonments – which compromise the effectiveness of oral PrEP over the long term.
Lenacapavir, marketed under the name Sunlenca®, is a new capsid inhibitor with a long half-life and strong antiviral potency. It has the advantage of opening a new class of antiretrovirals. Before its interest in injectable PrEP, it already represented a therapeutic advance, because it has no known cross-resistance with other classes of antiretrovirals. Lenacapavir is used as a last resort treatment in HIV-positive patients, in combination with other antiretrovirals. It is also currently being evaluated in combination treatment with broad-spectrum neutralizing antibodies (bNAbs).
PURPOSE 1 and 2
PURPOSE 1 involved 5,338 cisgender women aged 16 to 25, spread across 25 sites in South Africa and 3 in Uganda. The participants were randomly divided into 3 groups, the first receiving lenacapavir injection every six months, and the other two a version of oral PrEP once a day, Descovy® (emtricitabine 200 mg and tenofovir alafenamide 25 mg; F/TAF) or Truvada® (emtricitabine 200 mg and tenofovir disoproxil fumarate 300 mg; F/TDF).
The results, presented at the AIDS 2024 conference last July, and since published in the New England Journal of Medicinereported a 100% reduction in HIV risk compared to expected incidence, with zero cases of HIV infection among the 2,134 women in the lenacapavir group (an incidence rate of 0.00/100 people- years). On the other hand, 16 cases of HIV infection were observed among the 1068 women in the Truvada® group (incidence rate of 1.69/100 person-years) and 39 cases of HIV among the 2136 women in the TAF/FTC group, i.e. an incidence rate of 2.02/100 person-years. Rate related to poor observance Therapeutic compliance corresponds to strict compliance with the prescriptions and recommendations formulated by the prescribing doctor throughout a treatment, essential in the case of anti-hiv treatment. (We also speak of adhesion or adhesion.) oral PreP, as already reported in this population.
The effectiveness of lenacapavir was subsequently confirmed in men who have sex with men and trans women by results from PURPOSE 2, presented by Colleen Kelley of Emory University at the 5th HIV Prevention Research Conference (HIVR4P 2024), in Lima, Peru.
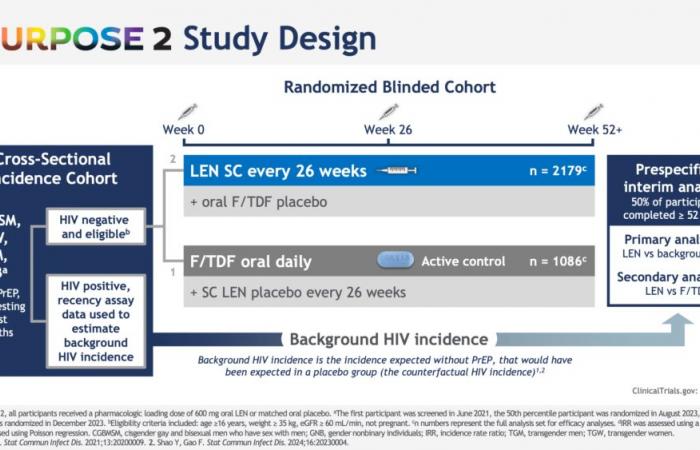
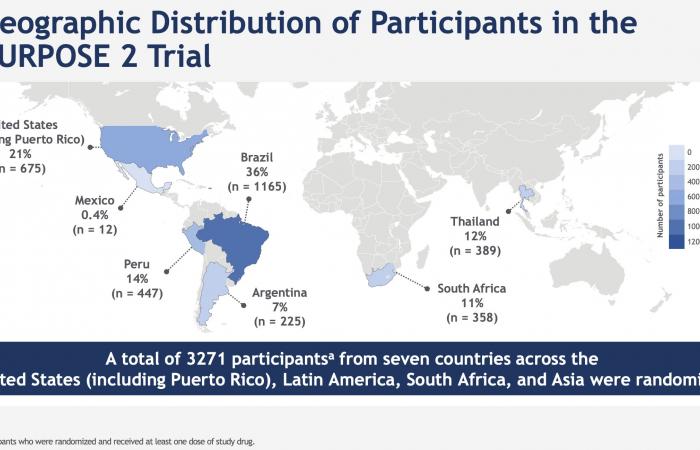
PURPOSE 2 was conducted with HIV-negative cisgender gay men, bisexual men or trans women, trans men and non-binary people in 7 countries in Argentina, Brazil, Mexico, Peru, South Africa, Thailand and in the United States.
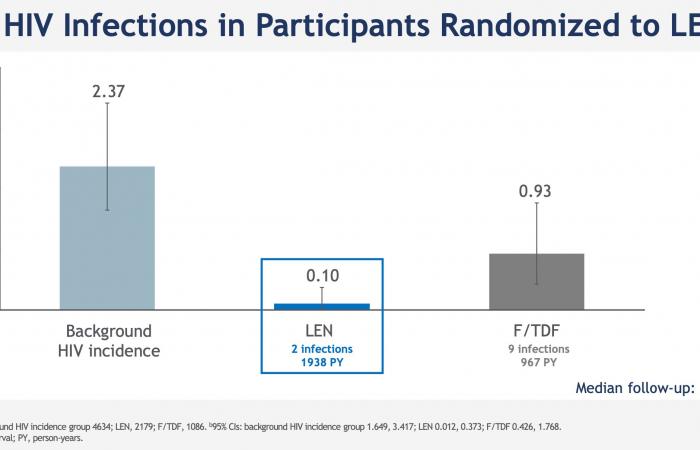
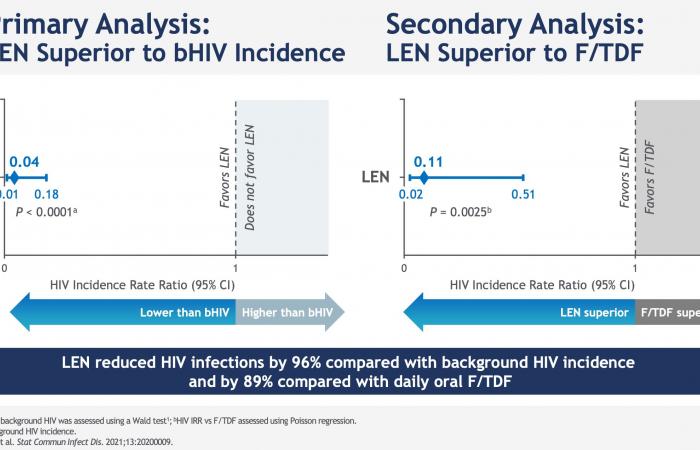
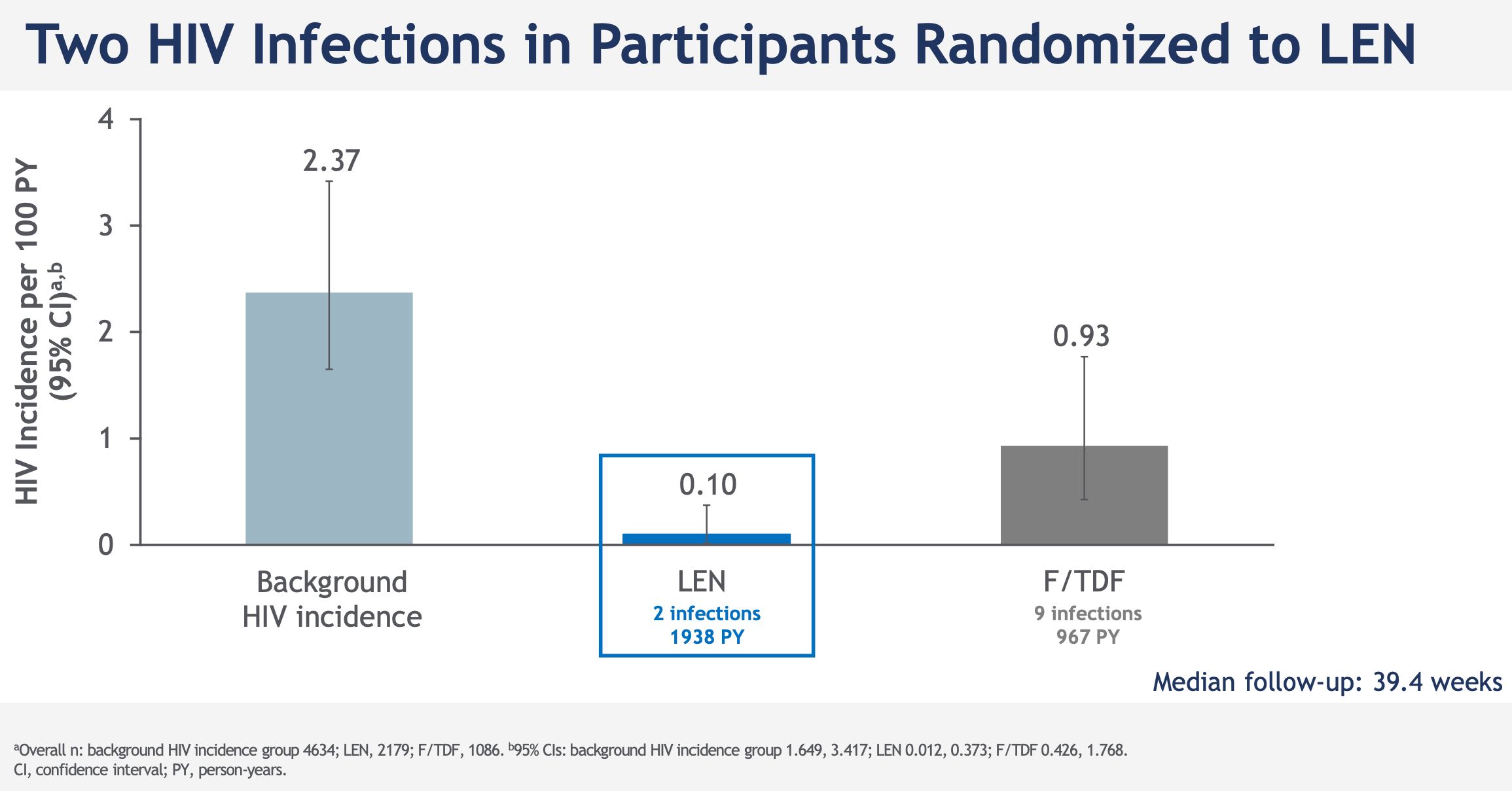
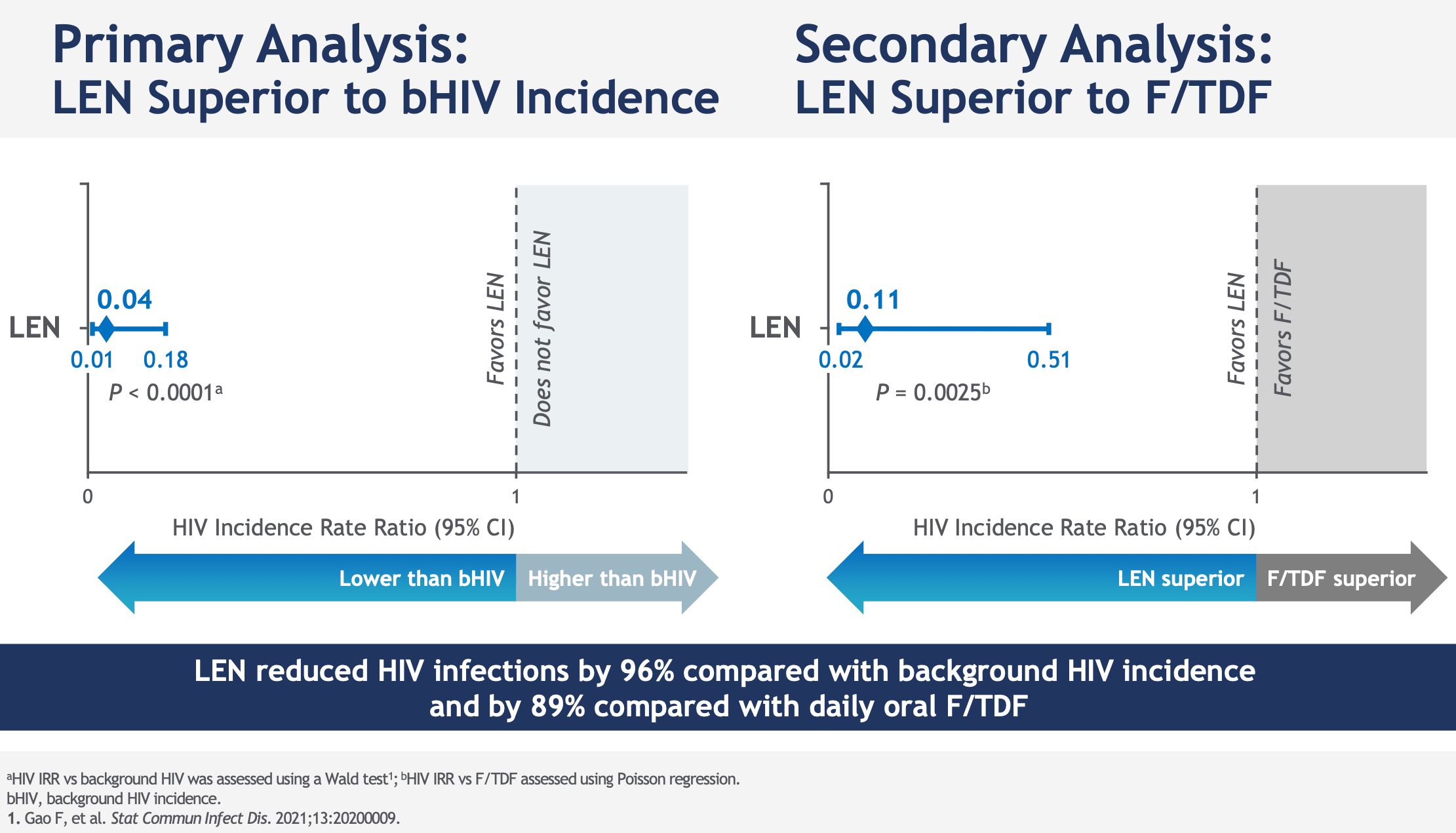
Among the 2184 study participants who received subcutaneous lenacapavir every six months, only two HIV infections were noted, an incidence of 0.10 per 100 person-years; 99.9% of participants did not contract HIV in the lenacapavir arm. Lenacapavir thus reduced HIV incidence by 96% compared to the expected incidence in a similar population without PrEP and by 89% compared to the arm that took daily oral PrEP, in which 9 infections in 1087 people were observed.
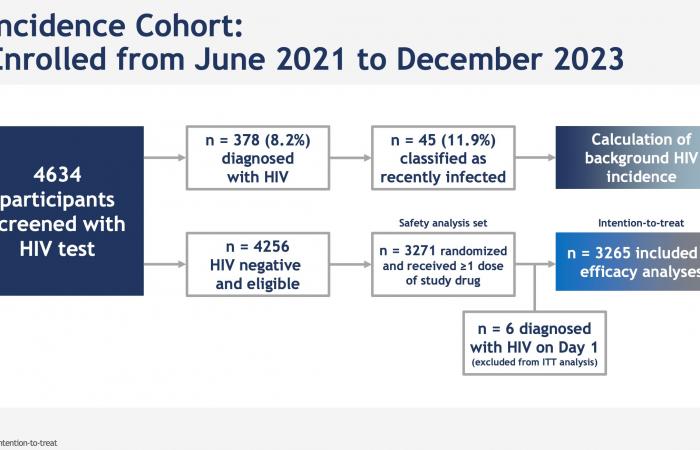
Note that each time, the effectiveness evaluated compares oral PrEP and injectable PrEP at an expected incidence: One arm placebo Inert substance, without pharmacological activity, having the same appearance as the product with which we wish to compare it. (Editor’s note: nothing to do with the alternative rock group formed in 1994 in London by Brian Molko and Stefan Olsdal.) would have been impossible to implement under ethical conditions, since the effectiveness of oral PrEP is now well established. However, at the screening for inclusion in the trial, the rate of infected men was very high, 378/4634 (11.9%, or 378 including 45 with recent infection) and 6 more were found infected with HIV. on the day the treatment begins. This is therefore the impact of PrEP in highly exposed populations.
What access and cost for lenacapavir
As Beatriz Grinsztejn, president of the International Aids Society (IAS), recalled at HIV4RP 2024 when presenting the results of PURPOSE 2, these results “confirm that lenacapavir in PrEP has the potential to transform the global landscape of HIV prevention for everyone, regardless of gender.
For key populations to benefit from this effectiveness, they must be able to hope to have equitable access to lenacapavir, currently marketed as a therapeutic for $40,000 per year. For Beatriz Grinsztejn, “all stakeholders must work together now, before regulatory approvals, to plan a rapid and equitable global deployment of this important new prevention tool.” On this subject, the IAS recently welcomed in a press release the measures taken by Gilead to expand access to lenacapavir, particularly in low-income countries.