Depending on the series, 5 to 10% of digestive hemorrhages originate in the small intestine, between the angle of Treitz and the ileocecal valve. They can be revealed by occult hemorrhage (iron deficiency anemia, positive blood test in the stools) or externalized bleeding (melena or red blood).
In the context of acute hemorrhage of small bowel origin (HAG), upper fibroscopy is negative and colonoscopy in practice is non-contributory when it is performed. The use of a video capsule is not suitable for the emergency context and it is generally a scanner with vascular time which localizes the bleeding at this level. A mesenteric arteriography can then be performed with a view to arterial embolization (EA) for therapeutic purposes.
There is little data available regarding the results of EA because many studies mix bleeding originating from the small intestine with those originating from the colon. Furthermore, we do not know the causes of failures or recurrences. A team from Louvain (Belgium) reports its experience of radiointerventional management of HAG.
An observational study on 31 patients with HAG and treated by arterial embolization
This was a retrospective study including patients with HAG and treated with EA between January 2006 and December 2021 in a tertiary care university center. Clinical, biological and imaging data were collected from medical records and radiological archives. After possible resuscitation, the patients had an upper endoscopy to eliminate bleeding upstream of the Treitz angle. They were then taken to the scanner for an examination carried out without then after injection of contrast product with arterial then portal phase.
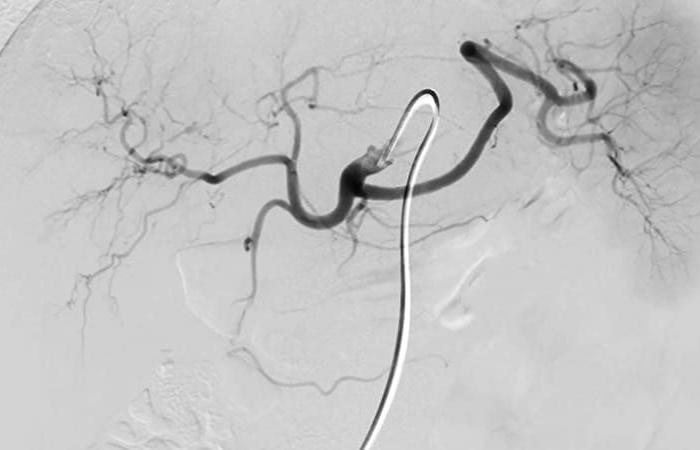
In the event of visible extravasation in the intestinal lumen or identified vascular lesion, and in the absence of hemodynamic instability, patients then benefited from an interventional radiology procedure using superior mesenteric arteriography for therapeutic purposes. Anesthesia could be local or general depending on the hemodynamic situation. Arteriography was performed via the femoral route with superior mesenteric catheterization using the classic Seldinger method.
In the event of detectable bleeding, hyper-selective catheterization was then performed using co-axial microcatheters. The material used for the EA could be micro-coilsmicroparticles or glue (mixture of Lipiodol and Histo-Acryl). Once the EA was performed, an angiographic control was carried out followed by a pressure dressing for 24 hours at the Scarpa level after removal of the catheter.
Clinical success assessed by the absence of persistent bleeding on D30 and without major complications
The technical success of the EA was defined by the occlusion of the artery responsible for the bleeding, while the clinical success of the EA corresponded to the absence of clinical or biological signs of persistent bleeding after 30 days and this without complications. major. Complications were classified as minor or major (according to the definitions of the Society of Interventional Radiology).
The Charlson index was used to assess the comorbidity score. In case of persistence of bleeding or recurrence, a multidisciplinary discussion made it possible to choose between a new EA, an endoscopic or surgical procedure or conservative treatment. A multifactorial logistic regression analysis was performed to identify risk factors for clinical failure and rebleeding.
Coagulation abnormalities or thrombocytopenia, associated with clinical failures or early recurrence of bleeding
A total of 31 patients were included in the analysis. More than half of the patients were recently operated on (16/31 = 52%) and 21/31 were even hospitalized at the time of the HAG (68%). Resuscitation maneuvers were necessary due to hemodynamic instability for 14 patients (45%) before radiological assessment.
Technical success was achieved in 97% of patients (30/31), and clinical success in 61% (19/31). Early recurrent bleeding was observed in 29% of patients (9/31). Four of them were treated with a new EA, while four others were operated on and one patient was managed palliatively. Major complications related to EA, including intestinal ischemia requiring surgical intervention, were observed in 6.5% of patients (2/31).
Thirty-day mortality was 19% (6/31), while overall hospital mortality was 23% (7/31). Actuarial survival at 5 years was 60%. In multivariate analysis, the presence of coagulation abnormalities (prothrombin time > 1.5, activated thromboplastin time > 45 seconds) or thrombocytopenia (platelets
An effective and relatively safe method but which depends on local expertise
EA is an effective and relatively safe method for treating severe small intestinal bleeding. The risk of intestinal necrosis is ultimately lower than one might fear, which is consistent with the data in the literature. This risk, without being completely eliminated, may be limited by hyper-selective catheterization and by the use of micro-coils. Finally, if early recurrence is common, almost ¾ of patients are definitively treated with one or two EA procedures.
The limitations of this study are of course its retrospective nature, although the emergency circumstances make it difficult to envisage a prospective study with randomization. The EA procedure itself is often dependent on the expertise of a single interventional radiologist. Furthermore, the authors only report the number of EA procedures actually performed and not the total number of angiograms performed for HAG. In addition, the angio-CT scan may be non-contributory at the time of its completion and bleeding may resume a few hours after its completion.