A new drug that slows the progression of breast cancer has been approved by Health Canada… but only for women.
The United States and the European Union have approved the use of this drug, Truqap, for both men and women, following a clinical trial.
Health Canada, questioned by CBC News, explained that the drug was only approved for the female population because too few men participated in the phase 3 clinical trial. (New window).
Only seven men took part, out of a total of some 700 participants.
For these men, the drug appeared to stop the spread of cancer for about two months, compared with about seven months for the overall study population.
The Canadian Drug Agency (CDA), for its part, reached a different conclusion from Health Canada regarding Truqap.
Its expert committee ruled that the drug should be reimbursed for all adult patients – men and women – under certain conditions.
The proportion of men in the study reflects the rate of breast cancer in men, theAMC, adding that it is impossible to say with certainty that the drug is less effective in male patients.
Breast cancer is common in women, but it is a rare disease in men, who account for only 1% of cases.
The Canadian Cancer Society estimates that by 2024:
-
30,500 Canadian women will be diagnosed with breast cancer;
-
5500 women will die from this disease;
-
290 Canadians will be diagnosed;
-
60 men will die from this disease.
Considering the rarity of the disease in men, it takes longer to collect the same amount of data for male patients.
In the case of Ibrance, another drug used to treat breast cancer, Health Canada authorized its use in men three years after it was authorized for women.
It doesn’t make any sense
says cancer specialist
Many doctors are calling on Health Canada to review its approval process for breast cancer drugs, starting with Dr. Philippe Bedard, an oncologist at Princess Margaret Cancer Center from Toronto.
Historically, men have been excluded from clinical trials testing new drugs. But what we now know is that the disease is really similar in men and women.
he explains.
Dr. Gerald Batist, director of the Segal Cancer Centre at the Jewish General Hospital in Montreal, echoed the same sentiment.
This is a very unusual cancer in men, but it behaves very similarly to breast cancers in women. We treat it in the same way as breast cancers in women.
The exclusion of Truqap for men has not no sense
insists the doctor, urging Health Canada to use his common sense and scientific reasoning
.
We have to look around the world and see that other panels, other regulatory agencies have approved this drug.
he says.
Health Canada does not want to expose anyone to undue toxicity. On the other hand, we have reached a point where we want better access to better drugs that will help people, and that is very urgent.
The federal agency, in its evaluation of Truqap, has indeed raised side effects such as diarrhea, skin rashes and nausea.
What is Truqap?
Clinical studies indicate that the drug, approved in Canada in January 2024, could prevent cancer from progressing for several months in patients with a type of advanced breast cancer (known as HR positive, HER2 negative).
This type of cancer responds to hormone therapy drugs and does not have abnormal levels of the protein HER2, which can accelerate tumor growth. Truqap stops the cancer from growing by blocking AKT, one of the enzymes needed for cell growth.
According to theAMCTruqap costs about $10,000 for a 28-day supply.
Side effects, such as those seen with Truqap, are common in treatments for advanced cancer. It is usually up to patients to weigh the pros and cons.
Open in full screen mode
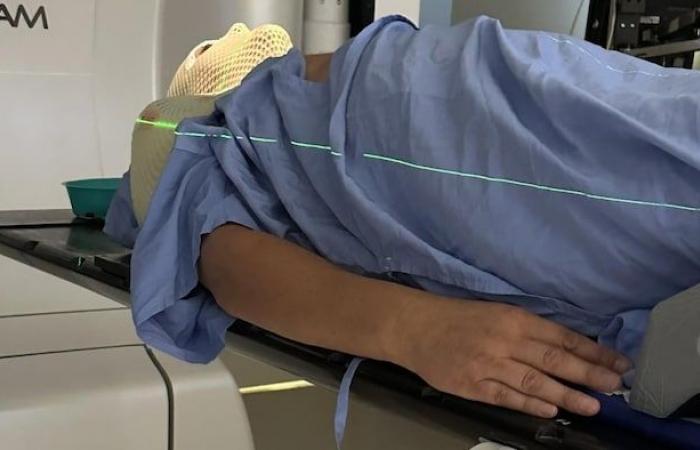
Warren Kotler has undergone several rounds of radiation therapy. But he fears his cancer is stronger than his treatments.
Photo: Warren Kotler
Well aware of the side effects of Truqap, Torontonian Warren Kolter regrets not having access to this new treatment.
When it comes to quality of life, I absolutely want to make an informed choice. But I have no choice with Truqap
he laments.
Mr. Kolter was diagnosed with stage 4 metastatic breast cancer eight years ago. He was given three to five years to live, but medication and radiation therapy helped beat his prognosis.
A father of three, Mr. Kolter wants to do everything he can to extend his life expectancy. I want to continue. I have a lot to do.
says the 61-year-old Torontonian, who hopes Health Canada will change its mind about Truqap.
According to a report by Jennifer Yoon, CBC News