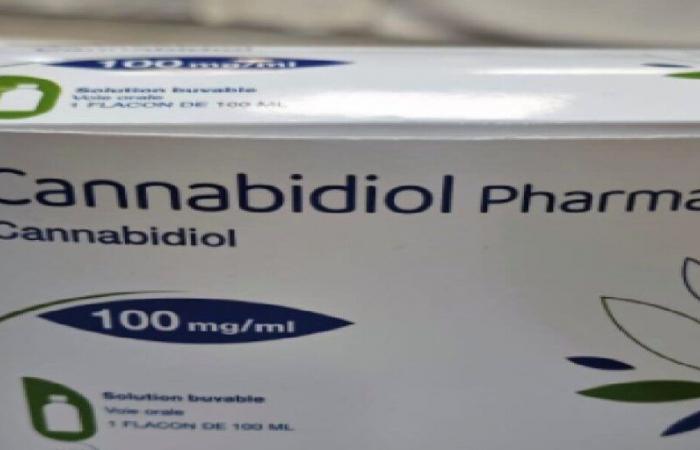
Pharma 5 announced the marketing of the first generic medicine based on therapeutic cannabis, 100% Moroccan.
Cannabidiol Pharma 5 is the code name of the new 100% Moroccan cannabis-based drug for intractable epilepsy, soon available on the market.
Intended for the treatment of intractable epilepsy, Cannabidiol Pharma 5 targets a pathology which affects around 100,000 Moroccans, mainly children, nearly 25% of whom do not respond to conventional treatments.
« This is a major step forward for us and for the country “, declared Mia Lahlou Filali, general director of Pharma 5, stressing that this drug obtained its marketing authorization (AMM) in September 2024.
Devoid of THC, the psychotropic molecule, it is presented as a safe and effective solution against this severe neurological disease.
The result of collaboration between the private sector and public authorities, the project required an investment of 250 million dirhams (around 23 million euros), including 25% dedicated to research and development.
« We have structured a fully integrated production chain, from the cultivation of the plant to the formulation of the finished product. », Specified Abdelmoumen Mahly, director of innovation and R&D at Pharma 5.
The marketing of Cannabidiol Pharma 5 is planned for the first half of 2025, after setting its price by the Directorate of Medicines and Pharmacy (DMP).
The drug should be prescribed by prescription and reimbursed by health insurance, similar to practices observed in other countries.
This launch places Morocco at the forefront of pharmaceutical innovation in the therapeutic cannabis sector, in accordance with the national strategy aimed at promoting this traditional resource for medical and economic purposes.
SL/ac/Sf/APA