Certificate of shared information, prescription by a specialist… From Monday, these medications based on valproate and derivatives, suspected of carrying risks for the fetus, will be more closely regulated.
Published on 06/01/2025 07:44
Updated on 06/01/2025 07:44
Reading time: 2min
From Monday January 6, France will once again tighten the delivery of anti-epileptic drugs, such as Dépakine. For years, the risks of malformations and neurodevelopmental disorders in babies whose mothers took these medications before and during pregnancy have been known.
But suspicion now also falls on fathers treated for epilepsy. It is in particular to better inform them that the Medicines Safety Agency is strengthening the conditions for prescribing these medications.
Since what we called “the Dépakine scandal“, the information is known: women who take anti-epileptic drugs based on valproate risk giving birth to children with malformations or neurodevelopmental disorders, that is to say autism and dys disorders. But since , men who also take these medications are wondering.
This is particularly the case of Jean-Marc Laurent. This father made the connection with the case of his daughter Margaux, now 16 years old, and suffering from cognitive disorders. “As a man, I said to myself, why wouldn’t it be transmitted from a man to a pregnant woman?” he explains.
And this is indeed what Marine Martin, whistleblower and president of Apesac, association of victims of Dépakine, supports. “We suspect an epigenetic phenomenon, therefore an impregnation via the sperm which will be transported into the womb of the future mother and which will poison the developing fetus”she explains.
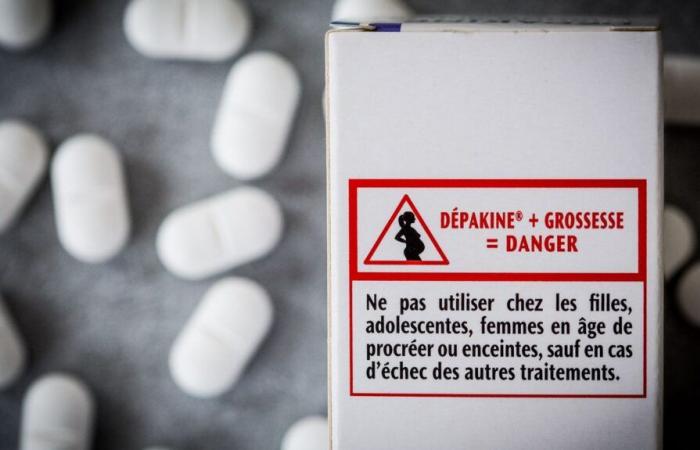
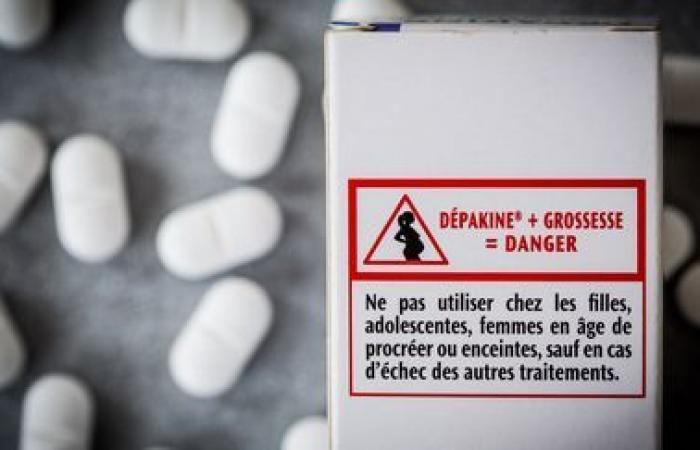
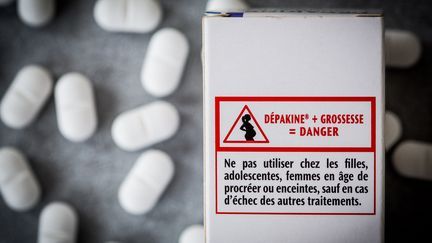
Although nothing is completely proven, a Scandinavian study shows that, statistically, men taking valproate have a greater risk of having children with neurodevelopmental disorders. The Medicines Safety Agency is therefore changing the conditions of access to these medicines for adolescents and men likely to have children. The first prescription must be made by a specialist, neurologist, psychiatrist or pediatrician and patients must be better informed, explains Marine Martin.
“There will be a shared care agreement form which will inform of this percentage of risk, a patient card stuck on the box of Dépakine, a booklet that the neurologist must give to the patient and he must inform him of the risks for the descendants and change his treatment if this man wishes to have children.”
Marine Martinat franceinfo
A notable step forward for Jean-Marc Laurent, this “Dapa Dépakine” who also campaigns within the Apesac association “So much the better if we can avoid it because it is eternal suffering for parents and children. If I had known it at the time, I would not have taken the risk of having children”he confides.
To date, in France, around 160,000 men take valproate-based medications.