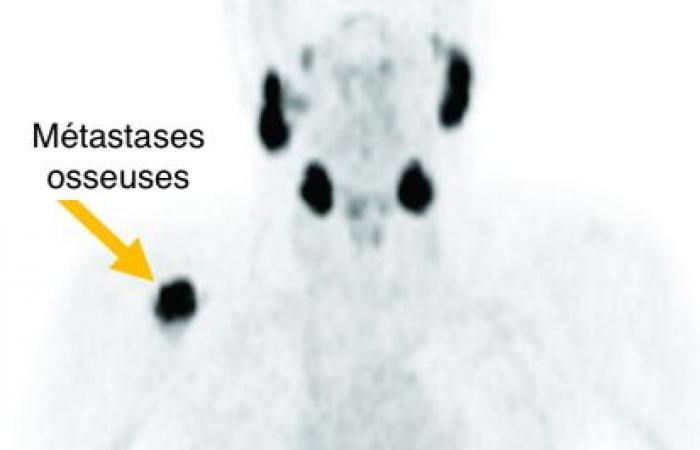
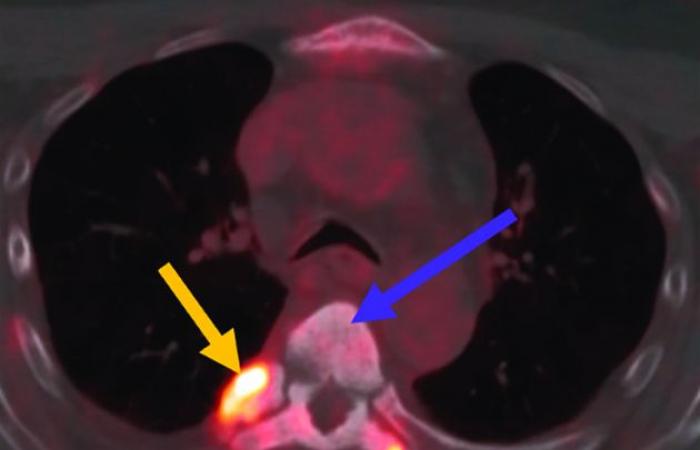
The treatment of metastatic prostate cancer is now done by intravenous injection of 177Lu-PSMA. This is a major advance against this metastatic prostate cancer, following an intravenous course every six weeks with essential specific monitoring and with emission of γ radiation which allows monitoring of the dose administered by scintigraphy.
Prostate cancer, in particular, metastatic castration-resistant prostate cancer (mCRPC) presents a significant therapeutic challenge. The diagnosis of this pathology, as well as the therapeutic arsenal to combat it, is now done by radiotheranostics of prostate-specific membrane antigen (PSMA) in nuclear medicine. There Revue Radiographics has just published an article which takes stock of the state of the art in the modern management of prostate cancer. He preambled that, until 2010, docetaxel was the only option available to manage patients with mCRPC, which is characterized by overexpression, amplification and mutations of the androgen receptor.
177Lu-PSMA injection, a major advance against metastatic prostate cancer
Following the implementation of various clinical trials, including the VISION trial, the Food and Drug Administration (FDA) approved the therapeutic agent 177Lu-PSMA, in March 2022, for patients who have progressed after treatment with chemotherapy . The molecular target is PSMA, which is highly expressed in more than 80% of prostate cancer cases. Diagnostic TEPScan targeting PSMA is used to confirm PSMA expression and assess suitability for treatment.
There are exclusion criteria, however, such as PSMA-negative visceral lesions measuring greater than 1 cm (e.g., liver or lung metastases), bone lesions with a soft tissue component greater than 1 cm, and metastatic lymph nodes greater than 2.5 cm.
An intravenous treatment every six weeks with essential specific monitoring
177Lu-PSMA, a radiopharmaceutical emitting β-minus radiation, is administered in one course intravenously at an activity of 7.4 GBq (200 mCi) every 6 weeks, up to a maximum of six courses, which can be discontinued early in the event of disease progression or significant toxicity. To best support the treatment, the patient must be sufficiently prehydrated with intravenous saline or oral hydration and must ensure good hydration during the first days following treatment. Blood tests within 5 days before each cycle and 3 weeks after each cycle are used to monitor bone marrow, kidney, and liver function.
In terms of toxicity, the organs in which the highest absorbed radiation doses are the lacrimal and salivary glands, the colon, the kidneys and the bladder wall. Patients are encouraged to increase fluid intake and urinate as often as possible to reduce radiation exposure to the urinary system. The most common side effects are fatigue, dry mouth and nausea, or, more seriously, myelosuppression, which can manifest as serious and life-threatening anemia, thrombocytopenia, leukopenia and neutropenia.
γ radiation which allows monitoring of the dose administered by scintigraphy
Assessment of treatment response should take into account clinical symptoms (e.g. bone pain, functional status), biochemical tests (e.g. PSA and alkaline phosphatase), as well as conventional and functional imaging. 177Lu has a half-life of 6.65 days, mainly emitting β-minus particles responsible for the therapeutic effect, but also γ radiation which allows post-therapeutic imaging, for monitoring the administered dose. Post-therapeutic SPECT/CT is used in this context for both qualitative and quantitative assessment, as well as a PSMA TEPScan within 3 months following the start of treatment, in order to monitor the appearance of possible tumors sometimes aggressive during this period.
The relevance of 177Lu-PSMA treatment is now being studied in the early stages of prostate cancer, so that metastases are no longer the only indications for this treatment. Ongoing trials are comparing this treatment to others conventionally used for primary prostate cancer, with patients having experienced improved quality of life and a lower rate of adverse events thanks to 177Lu-PSMA.
Bruno Benque