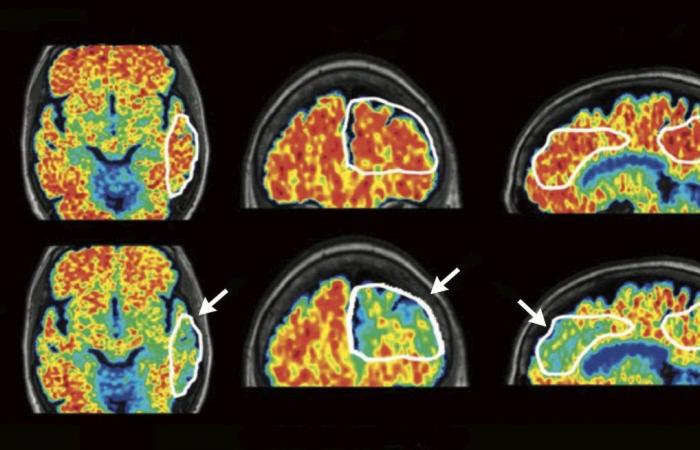
The diagnosis was made about three months ago following a positron emission tomography (PET scan) of the brain. Normand Viau’s small memory losses were indeed due to Alzheimer’s disease. Since this verdict, Mr. Viau’s family has placed great hope in lecanémab, the first drug to act on the causes of Alzheimer’s disease which, according to studies, slows cognitive decline by approximately 30%. The problem: this drug that was approved in the United States a year ago is still not approved here. Health Canada is still studying the file, which was submitted on June 10, 2023.
Mr. Viau is one of the patients who could receive lecanemab (trade name Leqembi), because his condition perfectly meets the eligibility criteria. First, he only has mild cognitive impairment, a sign that he is only at the very beginning of the disease. “My memory is starting to fade, but I am in good shape, I exercise every day,” he says in an interview. “We have to practice more often, but he is still as ingenious in all the little ‘patents’. It is especially in business that it has become more complex,” adds his wife.
Second criterion: the PET scan highlighted the presence of amyloid plaques, biological proof that “he is indeed suffering from Alzheimer’s disease, and not from dementia of vascular origin”, specifies the Dr Simon Ducharme, neuropsychiatrist and clinician-researcher at the Neuro and the Douglas Research Centre.
“There are other criteria that could make a patient ineligible for this treatment, including if they have other medical conditions or are taking other medications that could interact with lecanemab and put them at risk for complications. For example, patients taking blood thinners, blood thinners, will likely be excluded,” adds Dr.r Fadi Massoud, geriatrician at Charles-Le Moyne hospital and at the MoCA clinic.
According to a study published a few months ago, “there are less than 10% of patients [atteints de la maladie d’Alzheimer] which would be admissible for reasons of all kinds. So, even if the drug arrives on the market, its administration risks being restricted to a minority of patients,” summarizes the Dr Massoud.
A prohibitive cost
Furthermore, access to lecanemab will not be affordable to all eligible patients due to its prohibitive cost. The treatment, which normally lasts 18 months, could cost up to $30,000. To this will be added the costs of magnetic resonance imaging (MRI) examinations of the brain (approximately $1,000 each), which must be carried out periodically during treatment in order to check that the patient does not present cerebral edema or cerebrovascular accidents (CVA), two possible side effects of treatment and which, “in many patients, do not give symptoms”, underlines the Dr Massoud.
If such costs are not reimbursed by the Ministry of Health and Social Services (MSSS), many patients will not be able to cover them and, therefore, will not be able to benefit from treatment. This is the case of Sylvain Langlois, aged 57, who suffers from a familial form of Alzheimer’s disease (his mother died and his sister suffers from it). Mr. Langlois would be an ideal candidate for lecanémab treatment, according to the Dre Marie-Pierre Thibodeau, geriatrician at the CHUM cognition clinic, who sincerely hopes that her patient will be able to receive it.
Mr. Langlois’s wife also has great hope for this new treatment. “I’m so clinging to this medicine because my husband is so young. He took all the exams [PET scan, IRM] which are required to receive the treatment, he is ready to receive it. But it would have to be reimbursed because my husband does not have insurance,” she says with emotion.
Once the drug has received approval from Health Canada, it can be prescribed, but it must be evaluated by the National Institute of Excellence in Health and Social Services (INESSS), which “will recognize its value or not therapeutic and will recommend reimbursement or not,” recalls the MSSS. This evaluation process can take up to six months. INESSS has nevertheless already carried out consultations with patients, caregivers, associations and health professionals from the end of February to mid-April 2024, because the manufacturer had initially expressed its intention to file an application evaluation at the end of March 2024, but it ultimately postponed the submission of its submission, preferring to wait for Health Canada’s decision. “We keep all information transmitted by stakeholders for evaluation purposes,” said INESSS.
“With a view to a positive recommendation from INESSS, a negotiation of the price of the drug must follow by the MSSS with the manufacturer. It is therefore too early for the moment to comment on the possibility of reimbursing lecanémab,” replied the Duty the MSSS. For its part, Health Canada did not want to specify when it would make its decision: “Health Canada does not comment on the status of a drug under review. The time frame for completing the review depends on many factors, including, but not limited to, the need for additional data, discussions with the manufacturer, and requirements for updated safety information. A decision will be made once all required information has been carefully evaluated by Health Canada. »
All the doctors interviewed are in favor of government reimbursement, but all expect restrictions and very strict eligibility and exclusion criteria, including those stated above, and probably others, such as “doing the proof that the patient continues to benefit from the treatment after six months. There really needs to be a clinical impact associated with the removal of amyloid. However, this impact can vary from one patient to another,” underlines Dr.r Massoud.
“Right now, the manufacturer is promoting an 18-month treatment. But if most of the amyloid beta is gone in six to eight months, is there really any point in continuing treatment longer? The amyloid deposits probably took 20 to 30 years to build up in the brain, so once you remove them, it’s going to be another 30 years before they become problematic again. So there might not be much justification for extending treatment,” Dr.r Judes Poirier, deputy director of the Center for Studies on the Prevention of Alzheimer’s Disease at the Douglas Research Center.
“What is very important is what will happen after 18 months of treatment,” underlines Dr.r Some charm. If what we will see in the follow-up studies presents itself as a strictly one-time effect, that is to say that the treatment slows the progression by 30% because we have removed the amyloid, but that afterwards, the patients join the curve [de progression habituelle de la maladie], we will say to ourselves that it is not worth giving this treatment given its complexity. But if we see that the two curves continue to separate, and that at 30 months, it slows down by 50%, that completely changes the situation because, in this case, we will postpone accommodation in institutions,” he says. to be worth.
“It will only be necessary to be reimbursed for patients who will benefit the most and run the least risk,” summarizes the Dre Thibodeau.
One thing is certain, the Viau family is ready to assume the costs of the treatment at all costs. “We will try to get reimbursed by our personal insurance and we can always deduct a little from our taxes. As soon as it becomes available, let’s go ! » dit Mme Viau.
“I want to see my grandchildren grow up!” » says Normand Viau.