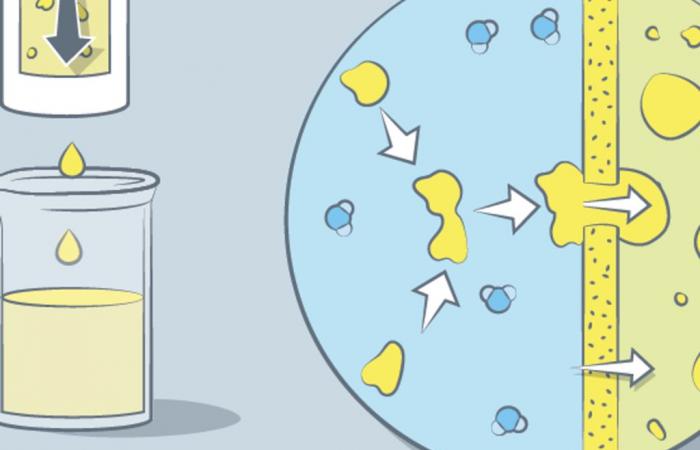
Oil and vinegar (or water) do not mix, but can coexist as an emulsion. In many industrial processes, it may be necessary to separate the two antagonistic products, for example, to treat effluents or recover products of interest. Current separation processes require either energy (centrifugation) or the addition of chemicals (coagulation). And, unfortunately, they only collect one of the products; the remainder to be reprocessed.
A Chinese team from Zhejiang University (Hangzhou) has developed an inexpensive process that separates the two in one go. She explains in the magazine Science of November 8 how she used two membranes with different properties, hydrophilic and hydrophobic, separated by 4 millimeters, to carry out the treatment.
“We discovered these membranes in 2014 and found a way to exploit them to achieve, for the first time, the separation of the two components of emulsions”explains Hao-Cheng Yang, first signatory of the study. Patents have been filed and industrial collaborations are planned. A business could be created.
Presence of surfactants
On one side of the canal, the water is separated. And on the other, it’s oil. The researchers observed that effectiveness depended on the hydrophobic and hydrophilic properties of their membranes, which are commercial polymer-based products, but also on the distance between them. A gap of 4 millimeters is twice as effective as a distance of 16 millimeters.
Read also | Article reserved for our subscribers The miracle recipe for mixing water with oil
Read later
Using such a technique may seem complicated, but everyone in their kitchen observes that the oil and vinegar will eventually separate on their own if you wait long enough. But, often, industrial emulsions are more complicated and more stable thanks to the presence of surfactants – also called surfactants – which keep the drops in suspension. Researchers have also shown that the greater the concentration of surfactants, the less effective their process is. But it is still better than if the channel was made of only a single hydrophilic or hydrophobic membrane. However, they also tested surfactants that nullified their efforts.
Senegal