⇧ [VIDÉO] You might also like this partner content
Using an improved version of AlphaFold, a protein structure prediction AI tool developed by Google DeepMind, researchers have identified a complex of three key proteins essential for egg-sperm recognition. This trimer would be common to all vertebrates and its absence inevitably leads to fertilization failure. This discovery contradicts the long-standing hypothesis according to which two proteins, located respectively on the two male and female gametes, are sufficient to ensure fertilization.
The life of any sexual organism begins with fertilization, namely the fusion of a sperm with an egg. The male gamete moves toward the egg cell guided by chemical signals and then binds to the egg surface through specific protein interactions. This interaction allows their membranes to fuse, then triggering the fusion of their genetic materials to form a zygote, the single cell that develops into an embryo.
However, despite advances in imaging, the molecular mechanisms governing this interaction remain partly mysterious. Indeed, unlike most cells in sexual organisms which avoid fusion and maintain a distinct genetic identity, the sperm and egg are specialized for fusion. This involves a sequence of precise and highly specific molecular events, taking place mainly at their lipid membranes. These processes are both discrete and fleeting, making them difficult to study with standard biochemical techniques.
On the other hand, the collection and storage of viable eggs and sperm in certain laboratory animals, such as mice, is particularly complex. Most studies of reproductive biology have therefore focused primarily on marine invertebrates (such as sea urchins), because they can release large quantities of eggs and viable sperm into the water.
To overcome these challenges, the researchers in the new study focused their work on zebrafish (Denmark rerio), a vertebrate that releases its ova (or eggs) and sperm into water. They also relied on the predictive power of AlphaFold, which won this year’s Nobel Prize in Chemistry for two of its developers, to determine in advance possible interactions between gamete membrane proteins.
AI has made it possible to uncover a previously unidentified essential protein. “ It’s no longer the old concept of having a key and a lock to open the door, it’s more complicated », Explains to the journal Nature Enrica Bianchi of the University of Rome Tor Vergata, who was not involved in the study.
Graphical summary of the study. © Victoria E. Deneke et al.
An additional sperm protein identified
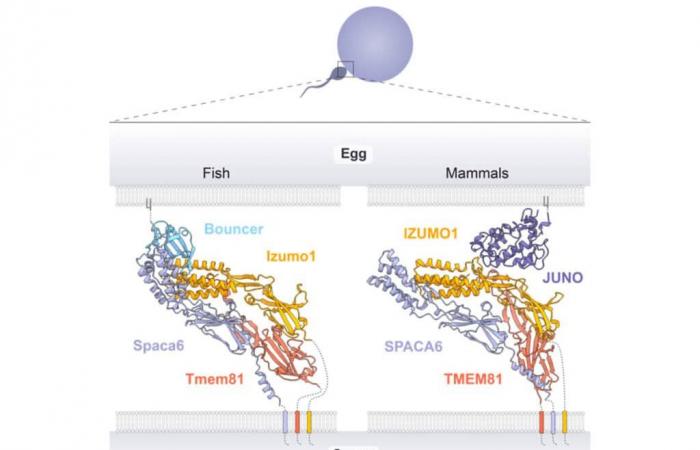
Over the past 20 years, genetic screening has identified several proteins essential for the interaction between male and female gametes of sexual organisms. Izumo1 is present at the sperm membrane and binds to Juno, which is present on the surface of the egg during fertilization. Other proteins structurally similar to Izumo1 (Spaca6 and Tmem95) are present at the sperm membrane, but do not appear to bind Juno, according to previous laboratory experiments.
Focusing their analysis on sperm binding proteins, the new study team used AlphaFold Multimer, an improved version of AlphaFold, to identify other potential binding proteins. “ We collected a list of proteins predicted to be present at the sperm membrane and performed a bioinformatics screen including thousands of predictions using AlphaFold Multimer », Explains in a press release from the Research Institute of Molecular Pathology (IMP), Victoria Deneke, co-senior author of the study.
The algorithm identified Tmem81, an additional previously unknown protein believed to be essential for Juno binding. Specifically, Izumo1 and Spaca6 interact not only with each other, but also with Tmem81 before they can bind to the egg protein. These first results demonstrate the importance of prediction and modeling algorithms in cases of limited empirical demonstration possibilities.
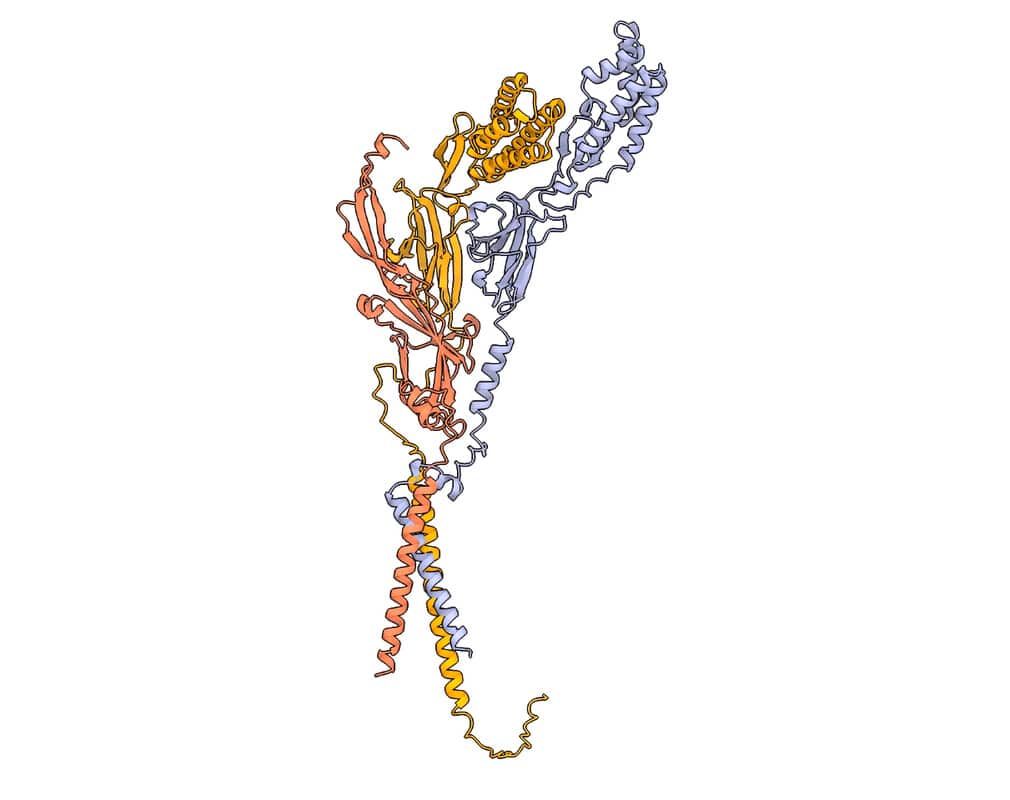

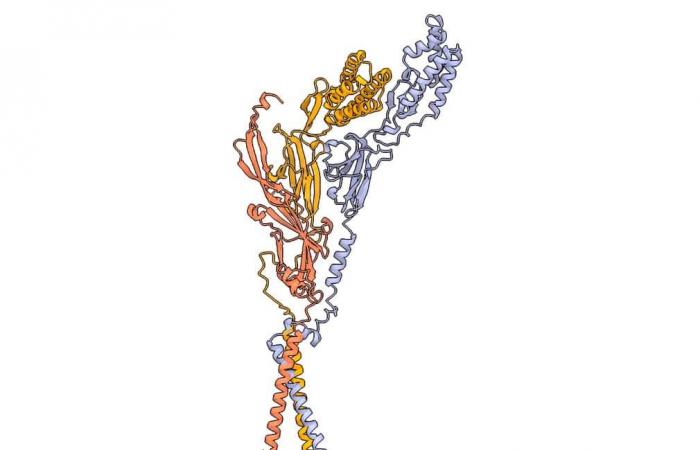
The trimeric sperm complex composed of Izumo1 (yellow), Spaca6 (lavender) and Tmem81 (red). © Victoria E. Deneke et al.
A complex conserved during vertebrate evolution
To test these predictions, the researchers observed the molecular processes of fertilization first in zebrafish, then in mice and in isolated human cells. They found that the trimer is indeed essential for fertilization in all models.
The complex of three proteins is anchored to the sperm membrane, while two of them constitute a binding site allowing the sperm to dock with the egg via Juno for mammals, and via an equivalent protein called Bouncer in mammals. Pisces. In other words, Juno and Bouncer serve as an access lock to the egg and only allow this access with the correct “key”. Furthermore, without trimer interaction, the male gamete is sterile and fertilization fails.
See also


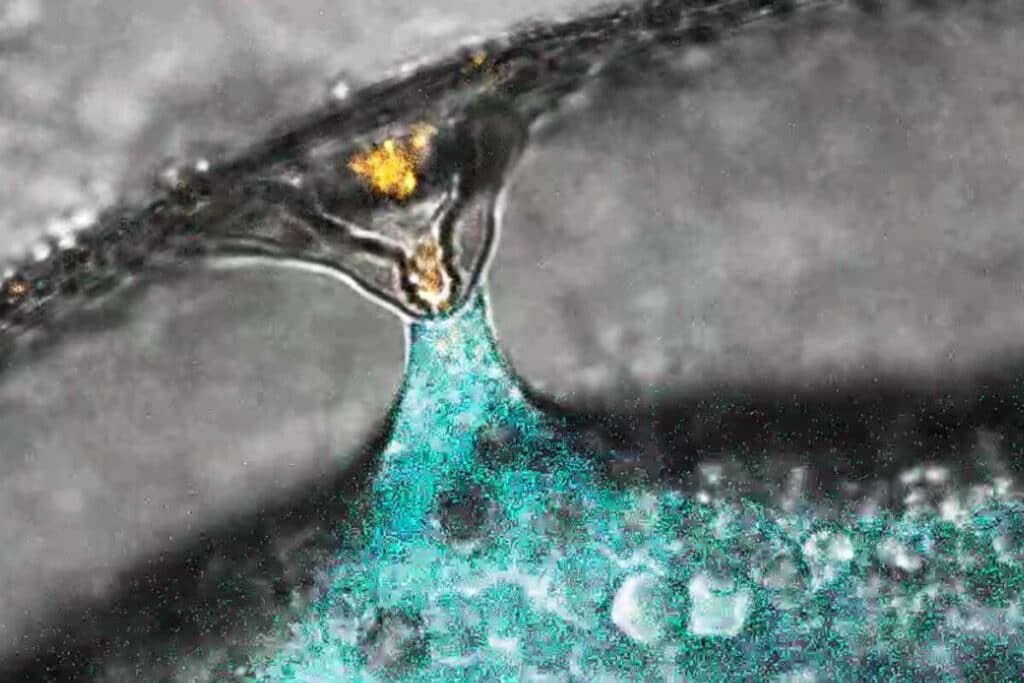

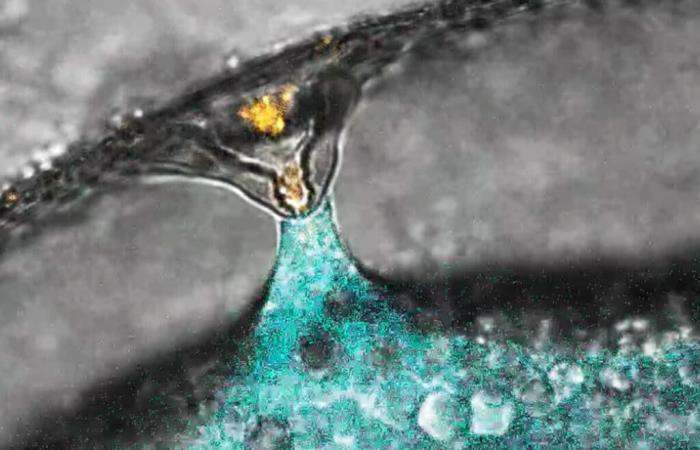
Fertilization of a zebrafish egg (marked in blue) by a sperm (marked in orange). © IMP
These results suggest that unlike egg proteins, the Izumo1-Spaca6-Tmem81 sperm complex is common to all vertebrates and has been conserved throughout their evolution. Juno comes, for example, from the duplication of a fetal receptor specific to mammals and is therefore only present in the latter. “ The fact that it has been maintained over millions of years of evolution shows how important this lock and key process is, but what is really surprising is that the conserved sperm trimer uses evolutionarily unrelated egg cell proteins to attach to the egg cell surface “, explains Andrea Pauli of the IMP, also co-author of the study.
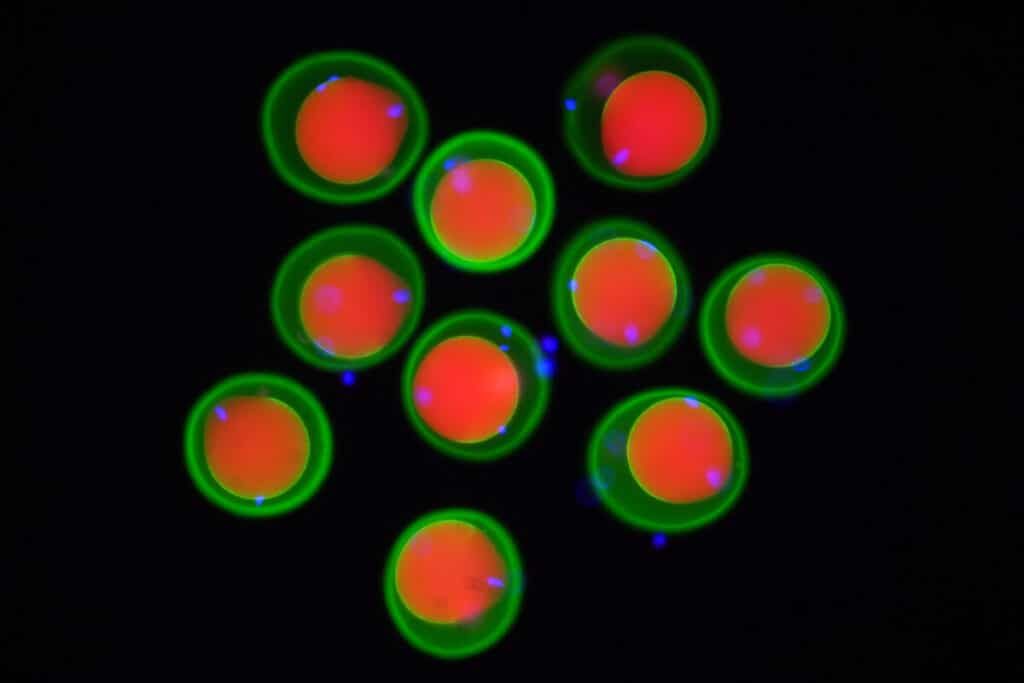

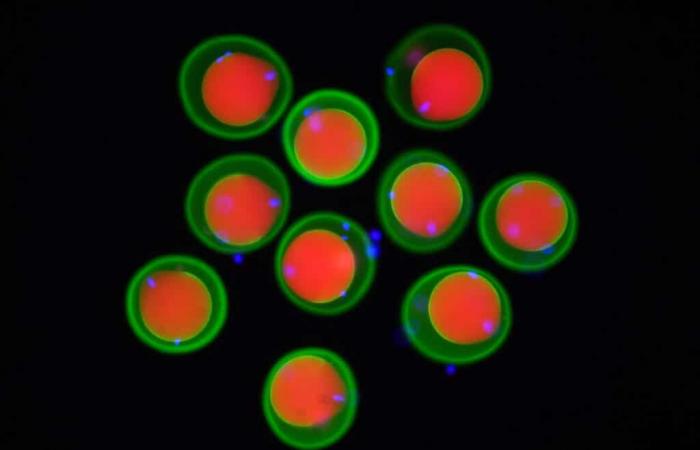
Image of mouse eggs (marked in red and green) and spermatozoa (marked in blue) obtained by fluorescence microscopy. © Yonggang Lu/Osaka University
This discovery could open the way to new screening techniques for fertility-related diseases. Understanding this molecular interaction could also potentially contribute to the development of new therapeutic interventions for infertility. The results of the study are published in the journal Cell.
Video presentation of the study: