A new deep learning model shows promise in the detection and segmentation of lung tumors, according to a study published in the Journal Radiology. The study results could have important implications for lung cancer treatment in the near future.
Accurate detection and segmentation of lung tumors from computed tomography (CT) is essential for monitoring cancer progression, assessing treatment responses, and planning radiotherapy. Currently, experienced clinicians manually identify and segment lung tumors on medical images, a time-consuming process subject to physician variability.
A deep learning model trained to detect and segment lung tumors using CT
Although deep learning methods have been applied to the detection and segmentation of lung tumors, previous studies have been limited by small datasets, a reliance on manual inputs, and a focus on segmenting single lung tumors. highlighting the need for robust, automated models capable of detecting and segmenting tumors in a variety of clinical settings.
In a study published in the Revue Radiology, A unique large-scale dataset consisting of routinely collected pre-radiotherapy simulation CT scans and their associated 3D clinical segmentations was used to develop a near-expert-level lung tumor detection and segmentation model. The main objective was to develop a model that could accurately identify and segment lung tumors on CT scans from different medical centers.
A model developed from more than 1,500 lung CT scans
“To our knowledge, our training dataset is the largest collection of CT scans and clinical tumor segmentations reported in the literature for building a lung tumor detection and segmentation model,” says the lead author. of the study, Dr Mehr Kashyap, from the Department of Medicine at the Faculty of Stanford University (California – USA).
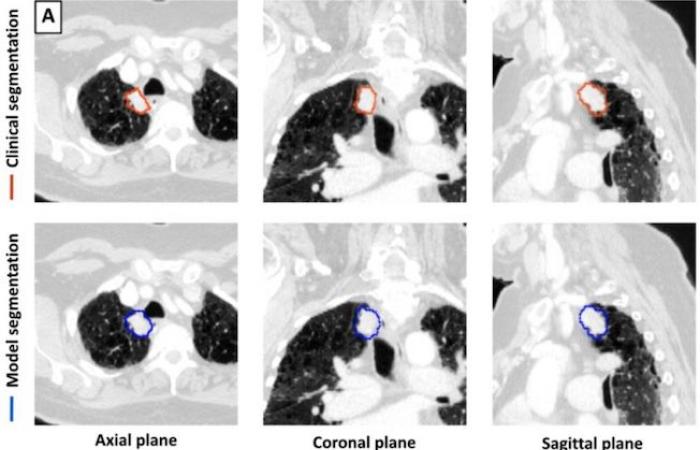
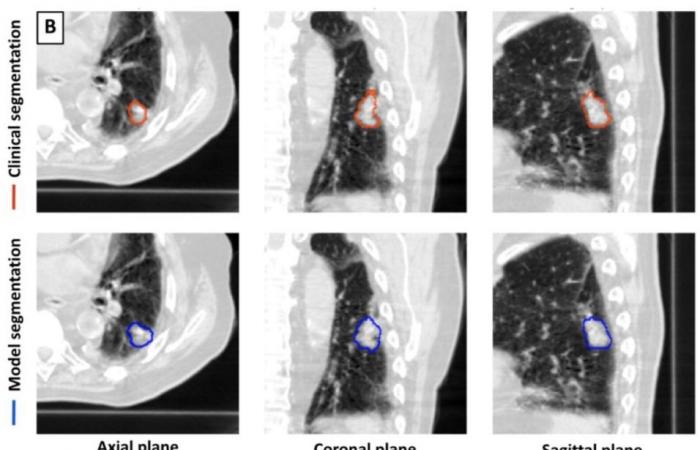
For this retrospective work, a 3D deep learning model U-Net was trained for lung tumor detection and segmentation using 1,504 CT scans with 1,828 segmented lung tumors. The model was then tested on 150 CTs. The tumor volumes identified by the model were compared to the volumes delineated by the physician. Performance measures included sensitivity, specificity, false positive rate, and Dice Similarity Coefficient (DSC).
Interesting performances of the model considered for tumor segmentation
-DSC calculates the similarity between two data sets by comparing the overlap between them. A value of 0 represents no overlap while a value of 1 represents perfect overlap. The model segmentations were compared to those of the three physician segmentations to generate model-physician DSC values for each pairing. The model achieved a sensitivity of 92% (92/100) and a specificity of 82% (41/50) in detecting lung tumors on all 150 CT scans combined.
 For a subset of 100 CT scans with a single lung tumor each, the median CBFs of model-doctor and doctor-doctor segmentation were 0.77 and 0.80, respectively. The segmentation time was shorter for the model than for the doctors. Dr. Kashyap believes that using a 3D U-Net architecture in model development provides an advantage over approaches using a 2D architecture.
For a subset of 100 CT scans with a single lung tumor each, the median CBFs of model-doctor and doctor-doctor segmentation were 0.77 and 0.80, respectively. The segmentation time was shorter for the model than for the doctors. Dr. Kashyap believes that using a 3D U-Net architecture in model development provides an advantage over approaches using a 2D architecture.
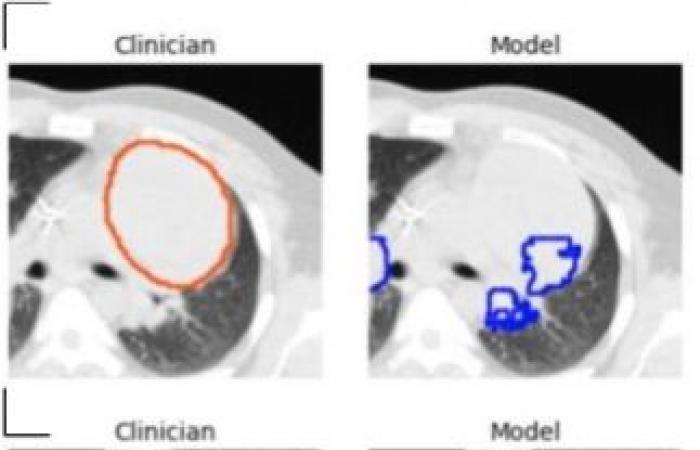
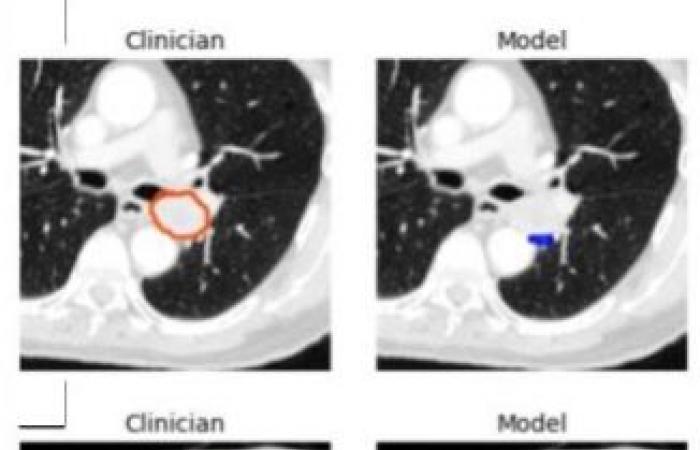
A tool to be refined, particularly in the presence of large tumors
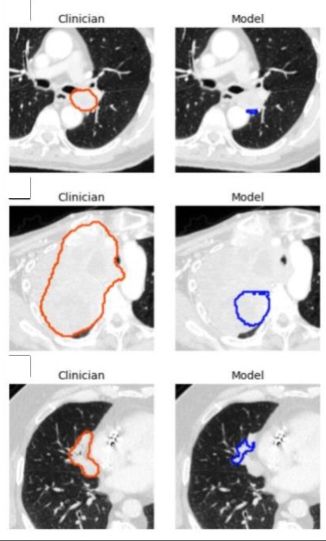 “By capturing rich inter-slice information, our 3D model is theoretically capable of identifying smaller lesions than 2D models which are unable to distinguish structures such as blood vessels and airways,” he adds. . One limitation of the model was its tendency to underestimate tumor volume, leading to poorer performance on very large tumors. For this reason, Dr. Kashyap cautions that the model should be implemented in a physician-supervised workflow, allowing clinicians to identify and eliminate misidentified lesions and lower-quality segmentations.
“By capturing rich inter-slice information, our 3D model is theoretically capable of identifying smaller lesions than 2D models which are unable to distinguish structures such as blood vessels and airways,” he adds. . One limitation of the model was its tendency to underestimate tumor volume, leading to poorer performance on very large tumors. For this reason, Dr. Kashyap cautions that the model should be implemented in a physician-supervised workflow, allowing clinicians to identify and eliminate misidentified lesions and lower-quality segmentations.
A new approach that could have many clinical implications in the future
The researchers further suggest that future research should focus on applying the model to estimate total lung tumor burden and assess treatment response over time, comparing it to existing methods. They also recommend evaluating the model’s ability to predict clinical outcomes based on estimated tumor burden, particularly when combined with other prognostic models using diverse clinical data.
“Our study represents an important step toward automating the identification and segmentation of lung tumors,” concludes Dr. Kashyap said. This approach could have broad implications, including its integration into automated treatment planning, quantification of tumor burden, assessment of treatment response, and other radiomics applications. »
Bruno Benque