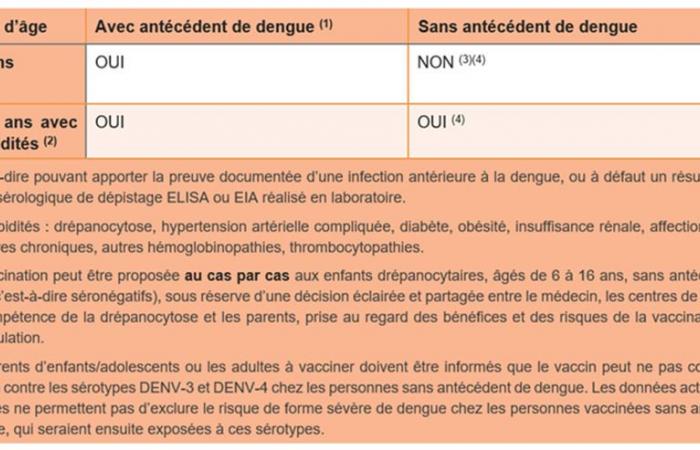
The Qdenga vaccine (Takeda) obtained European Marketing Authorization in 2022, for ages 4 and over. Data in children and adolescents having been previously infected with the dengue virus show the effectiveness of the vaccine in preventing symptomatic disease and hospitalizations for the 4 serotypes. This effectiveness ranges from 51.8% against DENV-3 to 80.2% against DENV-2 (but it is lower in children aged 4 to 5 years). On hospitalizations, the effectiveness is 98% against DENV-2 and 72% against DENV-1 and DENV-3.
On the other hand, among people who have never been infected with dengue (HIV negative)the vaccine showed a lack of effectiveness against serotypes DENV-3 and 4 in the prevention of symptomatic dengue, as well as against serotype DENV-3 in the prevention of hospitalizations. Due to a low number of cases linked to serotype DENV-4, effectiveness on this serotype could not be determined.
The available data shows good tolerance of the vaccine regardless of serological statusbut we cannot exclude, to date, the risk of developing severe forms of dengue in vaccinated seronegative people who would subsequently be exposed to serotypes DENV-3 and DENV-4.
The Qdenga vaccine is contraindicated in immunocompromised individuals and pregnant and breastfeeding women.
Health