Heralded as the revival of vaccination, the deployment of messenger RNA vaccines during the Covid-19 pandemic made their manufacturers successful on the financial markets. Four years after this phase of strong growth, a new bullish sequence is looming after approval from the American drug agency…
After decades of gestation in the laboratory, the Covid-19 pandemic was an opportunity for messenger RNA vaccine technology to arrive on the market.
The coincidence of the calendar brought together health emergency and technical maturity, allowing the design of Western vaccines based on this new platform which could, otherwise, have remained a biochemical curiosity.
If the anti-Covid messenger RNA vaccines did not have the effectiveness hoped for by the most optimistic who saw them as a way to eradicate the new virus, their performance was similar, or even superior, to alternatives based on adenoviruses.. And even their side effects, although measurable, ultimately remained acceptable. HAS title comparison, it is now estimated that the AstraZeneca vaccine, at the time deemed safer because it was not based on messenger RNA, was linked to thrombosis at a rate of 1 per 100,000 injections, and to deaths in 2 to 4 cases per million injections.
Thanks to the pandemic, messenger RNA vaccines have reached the milestone of large-scale production (more than 13 billion doses have been injected), while making it possible to collect an unprecedented amount of data in the history of modern medicine.
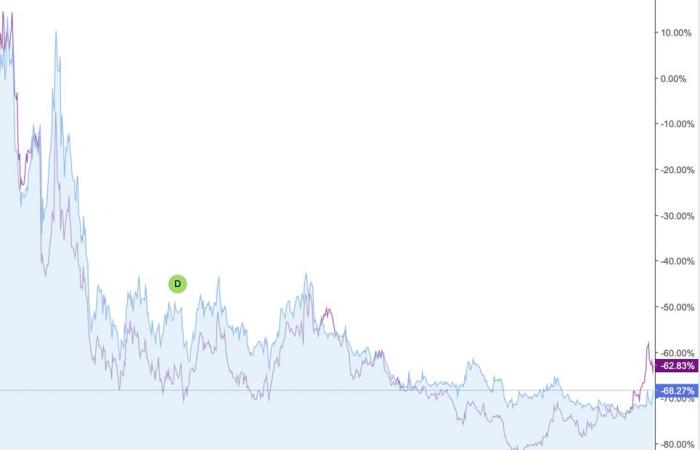
One piece of news chasing another, the Stock Exchange has since abandoned the shares of Moderna and BioNTech, causing them to lose up to -80% of their value at the end of 2023 compared to the peak of fall 2021.
Evolution of BioNTech (blue) and Moderna (purple) shares. The stock market has completely abandoned mRNA companies. Infographic: Investing.com
Now far from the spotlight, mRNA technology has nonetheless continued to evolve over the past two years. The promise of a variation of vaccines for other indications is about to be kept. From next winter, mRNA specialties could be part of the vaccine arsenal. Ultimately, messenger RNA could even be used as a curative treatment, and succeed where CRISPR/Cas9 never managed to break through.
FDA validates Moderna’s new vaccine
For two years, the markets have been worried about the ability of biotechs specializing in mRNA to reproduce their success. Caution was justified: outside of a crisis period, laboratories cannot rely on the simplified marketing procedures from which they benefited in 2021.
However, the world of health is clear: to make money, having a high-performance product is not enough. You also need gigantic financial reserves, armies of lawyers, and time… all these elements that Moderna and BioNTech lack, even after their success two years ago.
In fact, between 2022 and 2023, the turnover of Moderna collapsed. With the end of mass vaccinations, it went from $19.3 billion to $6.8 billion. Its rival BioNTech fared even less favorably, with revenues contracting from $17.3 billion to $3.8 billion.
But the crossing of the desert could well come to an end more quickly than expected. A few days ago, Moderna received the green light from the FDA for the marketing of its new specialty: mResvia. This new vaccine against respiratory syncytial virus (RSV, which causes bronchiolitis) is intended for older people. The stakes are high: among those over 80, mortality from bronchiolitis is similar to that of the flu.
The good news is back
This stamp from the FDA is a breath of fresh air for Moderna, which experienced a setback at the start of the year for its flu vaccine candidate. During the Phase III clinical study, the results of which were published in February, it proved disappointing against type B strains, the most common, while causing more side effects than traditional vaccines.
But no matter, the laboratory has not abandoned its flu vaccine project. If the mRNA-1010 does not present any major therapeutic interest, its little brother mRNA-1018 is currently being evaluated against a pathology that could become a major public health problem: avian flu.
According to the WHO, the H5N1 strain currently circulating across the planet has an average lethality of 52%, compared to 0.2% for seasonal flu. For the moment, all the humans affected have been contaminated by animals… but it would only take one unfavorable mutation for the virus to be transmitted from human to human. The health consequences would be out of all proportion to what we experienced four years ago.
It is therefore no surprise that health authorities are requesting therapeutic solutions in anticipation. According to Reuters, the US government is finalizing a subsidy component for test Moderna’s vaccine candidate, and would even provide an option to purchase doses in the event of success in clinical trials.
In the medium term, Moderna is using its war chest to fuel its pipeline. In addition to its vaccines against bronchiolitis (validated by the FDA) and against avian flu (ready to be tested), Moderna is also working on vaccines directed against certain tumors.
And In the long term, messenger RNA could be used as a drug for hereditary diseases. It would take over from gene therapies, which were not the miracle solution once hoped for.
Messenger RNA to make people forget CRISPR/Cas9
The promise of the CRISPR/Cas9 genetic scissors was great. By making it possible to directly modify our DNA, they opened the way to rewriting our genetic code to potentially resolve all hereditary diseases.
But in practice, genetic editing is more of a jackhammer than a scalpel. With current techniques, errors are numerous, leading to sometimes dramatic side effects. Gene therapies, by nature irreversible and still poorly understood, are therefore limited to the most serious pathologies. It is with caution that the FDA authorized, at the end of 2023, the first CRISPR treatment against sickle cell anemia.
In the case of diseases where genetic errors lead to the absence of production of a protein, messenger RNA offers an interesting alternative. Rather than reprogramming the genetic heritage of patients with all the risks that this entails, biotechs are working on basic treatments using messenger RNA. Once injected, it instructs cells to produce the missing protein. But as it is quickly destroyed, its effect is not persistent. In the event of no reaction to the treatment or excessive side effects, simply stop the injections for the patient to return to their previous state – a security impossible with genetic modifications.
Moderna is thus in the process of test treatment for propionic acidemia (PA). Concerning one in 35,000 births, it is characterized by neurological dysfunction and episodes of metabolic decompensation which put the vital prognosis at stake. Preliminary studies, on a reduced cohort of 16 patients, showed an improvement in blood markers in the majority. subjects.
Although well ahead of possible commercialization, these first tests will validate that messenger RNA is well tolerated in the long term and can be used more frequently than required for vaccine injections.
If this curative use were to emerge, messenger RNA experts would open up, after vaccines, an even more lucrative market: that of lifelong treatments.