This implant, which more precisely marries the forms of the cerebral trunk, makes it possible to reduce the activation of the non -targeted nerves and thus limits the side effects. Still in the research phase, it could represent in the future an alternative for patients who cannot benefit from a cochlear implant.
By Lucien Brenet, published on May 06, 2025
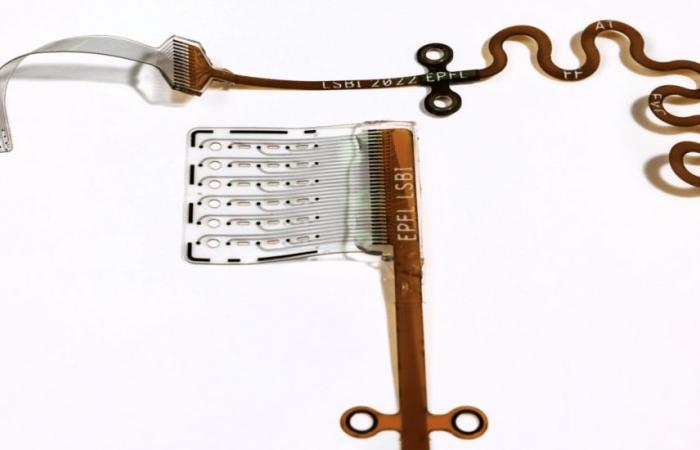
(C) 2025 EPFL/Alain Herzog The hearing implant of the flexible brainstem (ABI) developed at the EPFL marries the brain tissues, for more precise and better tolerated stimulation.
The Flexible Bioelectronic Interface Laboratory (LSBI) of the Federal Polytechnic School of Lausanne (EPFL) has developed a hearing implant of the flexible and ultra-fine cerebral trunk, designed for people whose auditory nerve is too damaged and which cannot benefit from a cochlear implant. Thick with a few millimeter fractions, the flexible matrix adapts to the shape of the brainstem, ensures better contact with neurons and thus reduces the activation of the undeveloped nerves, and therefore the side effects.
-Indeed, “The current ABIs are rigid and do not make good contact with the nervous tissue”describes the EPFL. With the consequence of side effects such as dizziness or facial contractions, which oblige to deactivate certain electrodes, reducing the effectiveness of the implant. “Consequently, most users of ABI only perceive vague, and little intelligible sounds when it comes to speech”, explains the EPFL.
Test the human system
For the time being, the device has been tested on macaques and has shown encouraging results, primates have also manifested no sign of discomfort, leaving hope for a future clinical application for human tests.
Before any marketing, the implant must still cross many research steps aimed at demonstrating its reliability, as well as various regulatory obstacles. The materials and components used must notably be approved for human use.
At first, clinical tests on humans are already possible, according to the researchers. The effectiveness of the implant could, for example, be measured as part of Abi interventions, temporarily inserting the flexible matrix with the implant.