Industrial facilities, such as those that make cement or steel, emit large amounts of carbon dioxide, a potent greenhouse gas, but the exhaust gases are too hot for carbon removal technologies. most modern carbon. It takes a lot of energy and water to cool the exhaust streams, which has limited the adoption of CO2 capture in some of the most polluting industries.
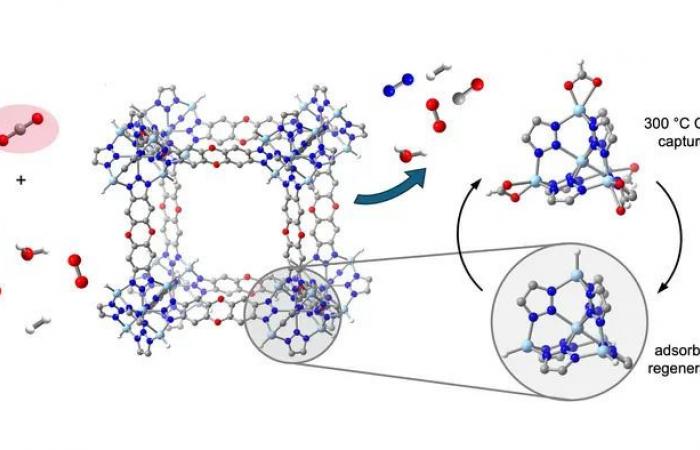
At center left is one of the crystal elements of a thermally stable metal-organic framework (MOF), called ZnH-MFU-4l, capable of reversibly and selectively capturing carbon dioxide, a greenhouse gas , from a mixture of many gases important to industry. CO2 is highlighted on the left, among molecules of nitrogen, oxygen, hydrogen, carbon monoxide and water. The MOF can capture CO2 for many cycles at 300°C, the typical temperature of cement and steel mill exhaust streams. The zinc hydride groups of the MOF reversibly bind and release carbon dioxide molecules (right). The light blue, gray, blue, red and white spheres represent Zn, C, N, O and H atoms, respectively.
Rachel Rohde, Kurtis Carsch and Jeffrey Long, UC Berkeley
Chemists at the University of California, Berkeley, have discovered that a porous material can act like a sponge to capture CO2 at temperatures close to those of many industrial exhaust streams. This material – a type of metal-organic framework, or MOF – will be described in an article to be published in the November 15 print edition of the journal Science.
The dominant method for capturing carbon from power plant or industrial emissions uses liquid amines to absorb CO2, but the reaction is only effective at temperatures between 40 and 60 °C (100-140 °F). Cement and steel manufacturing plants produce exhaust gases that exceed 200 C (400 F), and some industrial exhaust gases approach 500 C (930 F). New materials currently being tested, including a subclass of MOFs with added amines, decompose at temperatures above 150°C (300°F) or are much less effective.
“Expensive infrastructure is required to take these hot gas streams and cool them to the appropriate temperatures for existing carbon capture technologies to work,” said Kurtis Carsch, a postdoctoral fellow at UC Berkeley and one of the first two authors. of the article. “Our discovery is poised to change the way scientists think about carbon capture. We have discovered that a MOF can capture carbon dioxide at unprecedented temperatures – temperatures that are relevant to many carbon dioxide processes. CO2 emission This is something that was not previously considered possible for a porous material.
“Our work moves away from the predominant study of amine-based carbon capture systems and demonstrates a new mechanism for carbon capture in a MOF that allows for high-temperature operation,” said graduate student Rachel Rohde. from UC Berkeley and co-author of the first paper.
Like all MOFs, the material features a porous, crystalline network of metal ions and organic bonds, with an internal surface area equivalent to about six football fields per tablespoon – an enormous surface area for adsorbing gases.
“Thanks to their unique structure, MOFs have a high density of sites where it is possible to capture and release CO2 under appropriate conditions,” explains Carsch.
Under simulated conditions, the researchers showed that this new type of MOF can capture hot CO2 at concentrations corresponding to the exhaust streams of cement and steel manufacturing plants, which contain on average 20 to 30 percent CO2. , as well as less concentrated emissions from natural gas power plants, which contain around 4% CO2.
Removing CO2 from industrial emissions and power plants, after which it is either stored underground or used to make fuels or other value-added chemicals, is a key strategy for reducing greenhouse gases that are warming the Earth and changing the climate on a global scale. While renewable energy sources are already reducing the need for power plants emitting CO2 and burning fossil fuels, industrial plants that make intensive use of fossil fuels are more difficult to make sustainable, and flue gas capture is therefore essential.
“We need to start thinking about CO2 emissions from industries, like steel and cement manufacturing, that are difficult to decarbonize, because it is likely they will continue to emit CO2 even as our energy infrastructure shifts more and more towards renewable energies,” said Mr. Rohde.
Moving from amines to metal hydrides
Rohde and Carsch conduct research in the laboratory of Jeffrey Long, professor of chemistry, chemical and biomolecular engineering, and materials science and engineering at UC Berkeley. Mr. Long has been researching CO2-absorbing MOFs for more than ten years. In 2015, his lab created a promising material that was developed by Mr. Long's startup, Mosaic Materials, which was acquired in 2022 by the energy technology company Baker Hughes. This material contains amines which capture CO2; Next generation variants are being tested as alternatives to aqueous amines for CO2 capture in pilot plants, and as a means of capturing CO2 directly from ambient air.
But these MOFs, like other porous adsorbents, are ineffective at the high temperatures associated with many combustion gases, Carsch said.
Amine-based adsorbents, like those developed by Long, have been at the heart of carbon capture research for decades. The MOF studied by Rohde, Carsch, Long and their colleagues features pores decorated with zinc hydride sites, which also bind CO2. These sites have proven surprisingly stable, Rohde said.
“Molecular metal hydrides can be reactive and not very stable,” explains Rohde. “This material is very stable and allows for what is called deep carbon capture, which means it can capture 90% or more of the CO2 that it comes into contact with, which is really what you need for point source capture. Its CO2 capture capabilities are comparable to amine-based MOFs, but at much higher temperatures.
Once the MOF is filled with CO2, it can be removed, or desorbed, by lowering the partial pressure of the CO2, either by flushing it with another gas or by placing it under vacuum. The MOF is then ready to be reused for another adsorption cycle.
“Since entropy increasingly favors the presence of molecules like CO2 in the gas phase as temperature increases, it was generally thought that it was impossible to capture these molecules using a porous solid at high temperatures above 200°C,” Long said. “This work shows that with the right functionality – here, zinc hydride sites – rapid, reversible, high-capacity CO2 capture can indeed be achieved at high temperatures such as 300 C.”
Rohde, Long and their colleagues are studying variations of this metal hydride MOF to see what other gases they can adsorb, as well as modifications that will allow these materials to adsorb even more CO2.
“We are fortunate to have made this discovery, which has opened new avenues in separation science, focused on designing functional adsorbents that can operate at high temperatures,” said Carsch, who agreed a professorship in the Department of Chemistry at the University of Texas at Austin. “There are a considerable number of ways to tune the metal ion and binding element in MOFs, so it might be possible to rationally design such adsorbents for other high-temperature gas separation processes relevant to industry and sustainable development.