This text is part of the special notebook Relève en recherche
INRS master’s student Amira Becheikh manipulates the properties of the infinitely small to develop a remedy for water pollution.
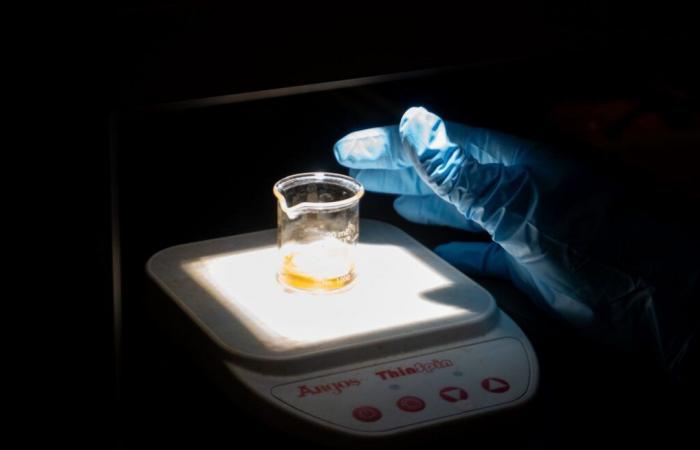
In a laboratory at the National Institute of Scientific Research (INRS), Amira Becheikh is busy destroying a bright orange solution to complete her master’s degree in energy and materials sciences. One could almost believe that her project, which takes advantage of the properties of nanoparticles, is that of an alchemist from ancient times. This is because his work, for ordinary mortals, seems to be impossible: how can such small fragments of matter be manipulated? For the student, nothing could be more natural: “My project uses perovskite nanoparticles to decompose pollutants,” she explains with a smile.
Amira Becheikh fell in love with materials science during her baccalaureate at the National Institute of Applied Sciences and Technology in Tunisia. “The field really fascinated me,” she says animatedly. An internship at INRS during her fifth year of studies allowed her to discover nanoparticles: “I was fascinated to learn how we can change and manipulate their properties according to our needs. » When Professor Andreas Ruediger offered her the chance to continue with her master’s degree, she jumped at the chance.
His work today allows him to work on the problem of contaminated water. “One of the good things about this technology is that it makes it possible to tackle a very wide range of pollutants,” she emphasizes, naming the dyes used in the textile industry, antibiotics or even the famous microparticles of plastic. “All kinds of things that are difficult to degrade at the moment. »
Particular particles
To fight against these pollutants, Mme Becheikh needs two ingredients: nanoparticles of perovskite, a mineral used in particular in the manufacture of solar panels, and an artificial “little sun”. Added to a polluted solution and exposed to the sun, the electrons of the perovskite “excite”, leaving behind “holes”, and interact with their environment, notably water and oxygen, a reaction called “oxidoreduction “. This reaction creates “reactive oxygen derivatives” which break down pollutants into less harmful substances, such as carbon dioxide or water. “The reaction makes it possible to separate the large molecule of the pollutant into smaller molecules,” summarizes the researcher.
All these reactions are made possible by the smallness of the perovskite particles, thousands of times smaller than a human hair. “The extremely small size of nanoparticles gives them unique properties, often very different from those of the same materials on a larger scale,” explains the researcher. Part of his master’s project also aims to determine the ideal size of perovskite particles to optimize their depolluting effect. “What I do is a surface reaction, so the more surface there is, the more reaction there is,” she notes. In this case, the smaller the particles, the more reaction surface they offer.
Her material of choice also offers a range of benefits that she attempts to exploit. “I’m trying to maximize the pyroelectric and piezoelectric properties of the perovskite,” she emphasizes. The first is linked to the ability of the ore to “generate an electrical charge when it undergoes a change in temperature”, while the second causes the perovskite to modify its electrical polarization when it is subjected to “a mechanical stress, such as the pressure or deformation.
Passion for research
For the moment, the researcher is carrying out her experiments with a bright orange dye, appreciated because it is “stable and inexpensive”. Its intense color also allows you to “see with the naked eye that it is deteriorating”.
Beyond the ability of its nanoparticles to attack pollutants, it will also have to determine the commercial potential of its research. “How do we move from the laboratory to a larger scale? » she illustrates. Not to mention that it will be necessary to ensure that the molecules produced after degradation do not cause new problems. “We want to know if they are toxic,” adds the student.
Passionate about these invisible particles, Amira Becheikh hopes to devote her career to research. Through a doctorate? “I haven’t made a decision yet, but I’m strongly considering it. »
This content was produced by the Special Publications team at Dutyrelating to marketing. The writing of the Duty did not take part.