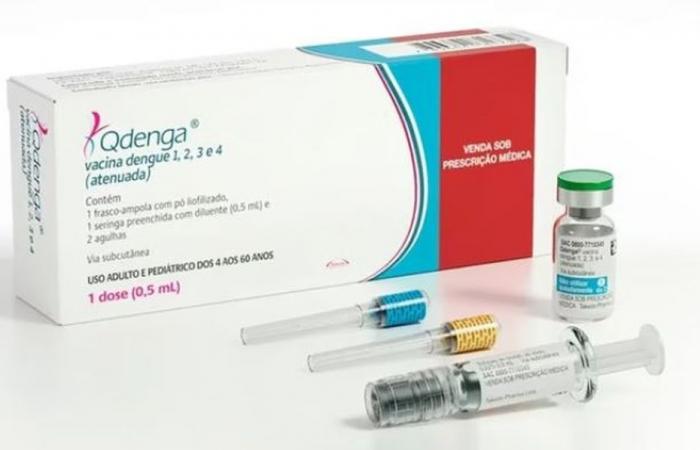
While dengue fever is more prevalent than ever in our territories, sometimes causing severe and fatal cases, the High Authority for Health published this Tuesday a vaccination recommendation for certain categories of populations overseas and in particular in the Antilles. It is the Qdenga vaccine, which has had European marketing authorization since 2022, which is recommended.
It was at the request of the General Directorate of Health that the High Authority of Health (HAS) evaluated the Qdenga vaccine. It has just published its conclusions this Tuesday, December 17, 2024 and recommends vaccination for certain categories of populations in the territories of the Antilles (Guadeloupe and Martinique), Reunion and Mayotte. Authorized on the European market since December 2022, this vaccine is now officially recommended for part of the population.
Are targeted for vaccination children and adolescents aged at least 6 years having already had dengue and adults aged 17 to 60 with risk factors (obesity, diabetes, sickle cell disease or other chronic illness, etc.). As a precaution, only children who have already had dengue will be affected by the vaccine. A serological test may also be prescribed in certain cases.
The recommended vaccination schedule consists of two doses of vaccine spaced 3 months apart. The need for a booster dose has not yet been established. The HAS recommends that the vaccination schedule be carried out during the inter-epidemic period. In the event of recent dengue infection, it is recommended to wait 6 months before giving the first injection of the Qdenga vaccine.
High Authority of Health
According to the HAS, subjects vaccinated with Qdenga must continue to apply individual protection measures with regard to mosquito bites (repellents, long clothing, mosquito nets, etc.); vector control remains an essential means of prevention in dengue control programs.
These HAS recommendations are addressed to public authorities. If you are interested in this vaccine, talk to your doctor.
Health
France