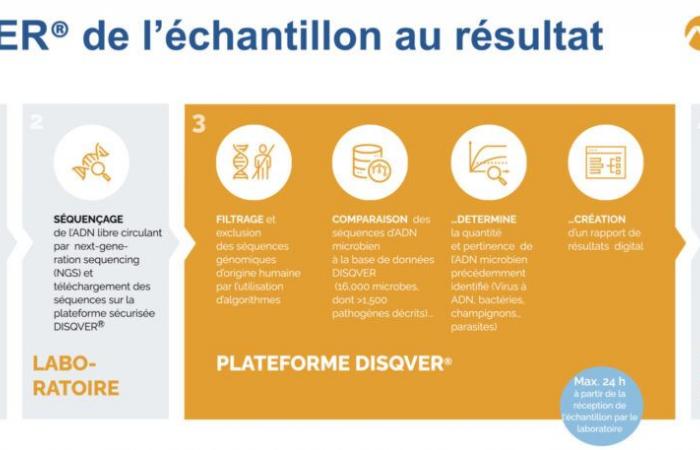
Noscendo is a company of around fifty people, born in Germany in 2017, and built from the work of doctors Silke Grumaz, Philip Stevens and Kai Sohn on the development of a complementary diagnostic platform for the detection of microorganisms by sequencing of next generation (NGS) of circulating free DNA from plasma samples of septic patients. The DISQVER solution® proposed today is therefore born from recent technological advances: next generation metagenomic sequencing and bioinformatics (computational capacity and improved algorithms in parallel with in-depth work on the qualification of the clinical database of listed pathogens).
Digital diagnosis
Frédéric Vigier, key account manager France and Benelux, of Noscendo.DR
Thus, Noscendo’s solution is a digital diagnostic platform that can identify 16,000 microorganisms, including 1,500 pathogens. The process, once the sample arrives at the laboratory, takes 24 hours. “ The result generated by comparing the raw data to process controls and an uninfected control population is communicated to the biologist in number of reads standardizedspecifies Frédéric Vigier, key account manager France and Benelux for the Noscendo subsidiary in France. We are the only ones today to offer CE-IVD marked technology for pathogen detection on whole blood. »
Although the use of free circulating DNA is now well developed in oncology, it is still not widespread in the context of infectious diseases for the search for pathogens. Thus, DISQVER® is a bioinformatics solution allowing the identification of the pathogen(s) in the event of coinfection, at the species level, from liquid biopsy (blood, synovial fluid or bronchoalveolar washing, among others). This is an agonist technique that allows the detection of bacteria, fungi, viruses and parasites.
For what indications?

Myriam Livrozet, Market Access Noscendo France / DR
« Today, metagenomics by high-throughput sequencing (mNGS) goes beyond a solution of last resortexplains Myriam Livrozet, Market Access Noscendo in France. Able to detect a wide range of pathogens in a single analysis, without prior culture or influence of ongoing treatments, where targeted techniques such as serology or PCR tests can fail. mNGS represents a powerful asset in the diagnosis of complex infections, in cases of rare or emerging pathogens, tedious or non-culturable culture. Its speed, precision and non-invasive nature make it an essential complement to traditional methods, allowing clinicians to obtain a comprehensive and reliable diagnosis to optimize patient care.. »
Frédéric Vigier adds: “ We are also relevant for cases where the syndromic approach is not possible, because there is no suggestive syndrome, but just a fever of unknown origin or febrile neutropenia. We can then rule out the infectious path or not and, if necessary, direct us towards the correct pathogen. Like personalized medicine in oncology, identification of the pathogen responsible for the infection allows the use of the appropriate antimicrobial. »
Limits?
“As with any diagnostic technique, you have to know its limits », underlines Frédéric Vigier. His colleague adds: “ Our solution does not yet give any indication of antibiotic resistance, for example. And on the differentiation between colonizing or commensal species from pathogenic species, we give indications, but for certain species, it is delicate. This indication is constructed by quantification indices (number of reads) – we have defined standards -, but it is up to the clinician and biologist couple to discuss the results in view of the clinical context. Our bioinformatics solution, however, erases a lot of the background noise thanks to control groups of different profiles already implemented (for example, immunocompromised patients). »
Solid data, controlled process
What about the competition? “ Today, many actors can do metagenomics. Our strengths lie in the solidity and clinical validity of our solutionexplains Frédéric Vigier. On the one hand, our protocol is rigorously supervised – a team of engineers travels to train the teams in order to ensure compliance and optimal control of the sample preparation and data generation process – and, on the other hand, , our database is private and verified, which guarantees the reliability of the results. In comparison, many other players use public clinical databases which are not always up to date and often poorly documented, particularly in myco- or parasitological matters. We now have years of experience and thousands of samples in our database, allowing us to further refine our processes and methods. »
« In Franceadds Myriam Livrozet, We are happy to have been chosen by the microbiology department of Brest University Hospital this year. This is a real mark of trust and recognition for our solution. »
Projected developments
Now present in Germany and France, Noscendo is currently considering an extension to the United Kingdom.
For France, the cost of diagnostic metagenomics procedures can be covered within the framework of the RIHN according to the indications of learned societies.
For its part, the company is continuing its R&D programs, in particular to explore the possibility of identifying antibiotic resistance, as well as its efforts to comply with CE-IVDR regulations and the extension of validated sample types. “ The difficulty lies in obtaining enough samples to validate the studies giving access to IVDRspecifies Myriam Livrozet. The transition to IVDR is a financial challenge due to the number of studies to be carried out to submit the file. »
The company also remains attentive to technical developments in sequencing and new equipment placed on the market.
What place in the clinicobiological journey?
Last resort or direct access? In certain cases, would it be more judicious to go directly through metagenomics? “It is a medico-economic and clinicobiological choice which, for the moment, has not yet been evaluated, the solution not being sufficiently democratized to carry out this type of study”, explains Myriam Livrozet. However, we note that certain specialties are showing growing interest in this approach. Thus, the International Society of Infectious Cardiovascular Diseases (ISCVID) has integrated the use of mNGS into its 2023 Duke criteria for the diagnosis of infectious endocarditis.