According to the presentation of Dr Koolawee Nademanee at the HRS 2025 congress in San Diego.
Key messages
- The brave study is the first prospective randomized work on this subject
- It shows the interest of removing the epicardial substrate in patients with Brugada syndrome in secondary prevention of ventricular rhythm disorders
- Ablation technique that appears safe when carried out in experienced centers
- Patients associating Brugada syndrome and early repolarization have a bad result of ablative management.
Introduction
Brugada syndrome is a well -known etiology of sudden death. The implantable automatic defibrillator is indicated in patients symptomatic of ventricular rhythm disorders. Pharmacological prevention means such as quinidine have shown significant but limited efficiency.
The removal of the epicardial arhythmogenic substrate of the right ventricle chamber emerges as an alternative solution in observational studies. The brave study had the ambition to confirm this hypothesis on a prospective randomized work.
Methodology and results
This is a prospective multicentric randomized study, conducted on a population of 52 patients (25 in the ablation of the epicardial substrate (2 patients having been excluded), 25 in the control group). The main criterion was a composite criterion of mortality all causes and FV episodes. Randomization was arrested prematurely due to an increased profit found in the Ablation group.
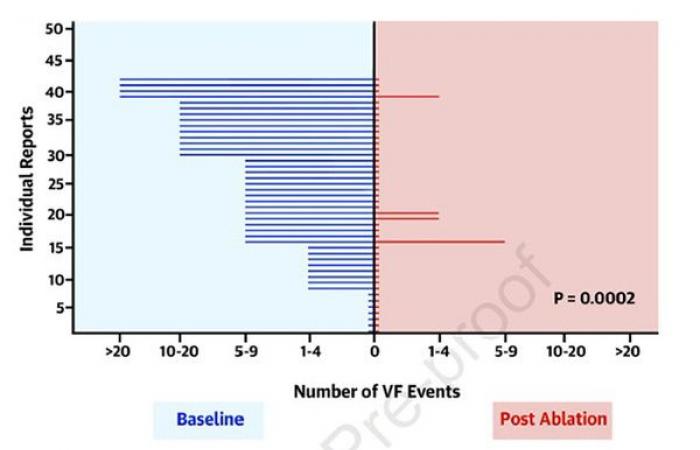
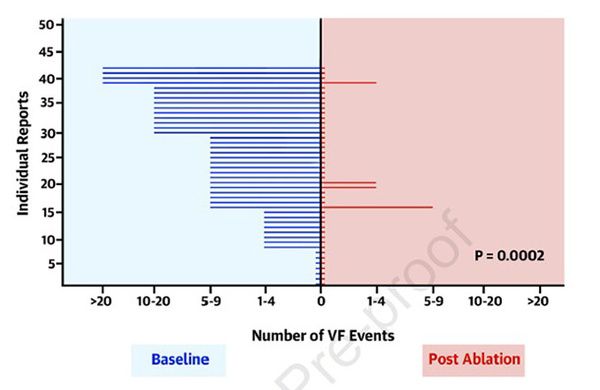
Results : The average follow -up was greater than 24 months. There are 18 episodes of FV in the VS 5 control group in the Ablation group (0.288, 95% CI: 0.102–0.811; p = 0.0184) (Figure1). In fact, a total of 42 patients benefited from removal (8 cross over and 9 patients who have declined randomization in addition to the 25 patients in the ablation group) with success for 38 patients (90%) (Figure 2) for an average of 1.1 =/-0.3 procedures. It was not noted by procedure or during follow -up, but a hemopericarde. Finally, patients with early repolarization associated with the aspect of Brugada and those whose ECG appearance persists post ablation have a more severe rhythmic prognosis.
Conclusion
The brave study is the first prospective randomized study highlighting the interest of specific removal in patients with Brugada syndrome symptomatic of ventricular rhythm disorder.
-
Figure 1

Figure 2
References
1. Nademanee K, Wongcharoen W, Chimparlee N et al. A Brugada syndrome ablation for the prevention of ventricular fibrillation episodes (BRAVE). Heart Rhythm. 2025 Apr 23:S1547-5271(25)02381-1. doi: 10.1016/j.hrthm.2025.04.033.
2. Santinelli V, Ciconte G, Manguso F, et al . High-risk Brugada syndrome: factors associated with arrhythmia recurrence and benefits of epicardial ablation in addition to implantable cardioverter defibrillator implantation. Europace. 2023 Dec 28;26(1):euae019. doi: 10.1093/europace/euae019.
All the news of the HRS 2025
All the news of French rhythm of SFC!