THE ESSENTIAL
- The ANSM suspends a clinical trial carried out by the promoter Ceraver on a new total hip prosthesis Actisurf-Cerafit.
- There are two reasons for this decision: adverse events and breaches of regulations governing research involving humans.
- Patient monitoring will be reinforced, with a notice period of 3 months for patients with an Actisurf-Cerafit prosthesis and 6 months for those equipped with a Cerafit reference prosthesis.
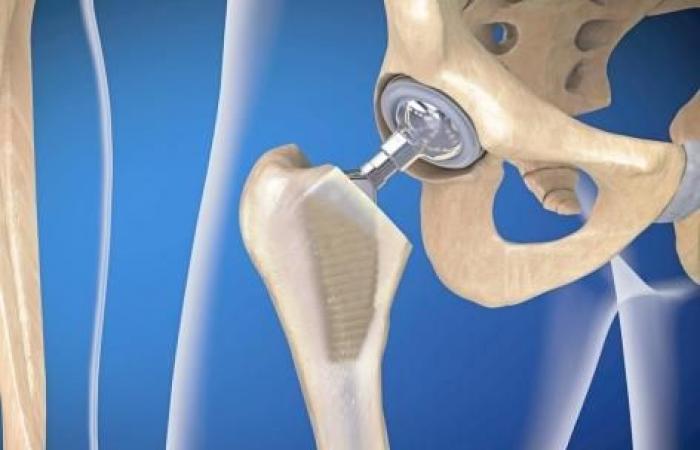
Osteoarthritis, osteonecrosis, femoral neck fracture, inflammatory disease, etc. Total hip prostheses are generally offered to patients who have cartilage destruction in the hip. There are several types, from various manufacturers.
ANSM suspends a clinical trial
One of them is currently in the sights of theNational Agency for the Safety of Medicines and Health Products (Ansm): Ceraver. The health authority has just suspended the “ACTISURF-OI-16” clinical trial, which concerns the Actisurf-Cerafit total hip prosthesis.
This is being carried out on humans, in seven surgical departments. Among the health establishments, there is in particular the orthopedic surgery department of the Ambroise Paré AP-HP hospital located in Boulogne-Billancourt, near Paris.
In total, approximately 340 patients were included in the research. They are randomly distributed between the new total hip prosthesis Actisurf-Cerafit and the reference Cerafit already marketed.
Adverse events on the new hip prosthesis
The ANSM puts forward two reasons to explain the suspension of the clinical trial:
- Adverse events, such as an increased risk of loosening of the new prosthesis in implanted patients, compared to the Cerafit reference prosthesis
- Breaches in the regulations governing research involving humans, including inadequacies in terms of the means implemented to ensure the quality of research data and patient safety.
“Therefore, we are taking a health policy decision against Ceraver to partially suspend this research, indicates the ANSM in a communiqué. This decision also implies that the sponsor informs, via the investigative centers, all patients of the reinforced monitoring methods.”.
Participants should soon be called – within 3 months for patients with an Actisurf-Cerafit prosthesis and within 6 months for those equipped with a Cerafit reference prosthesis – by the orthopedic surgeon and thus find out the type of hip prosthesis that has fitted them. been implanted.
“If you experience pain and/or a noise coming from your hip, contact your surgeon immediately so that he can check the condition of the prosthesis.”, recommends the ANSM.
Health