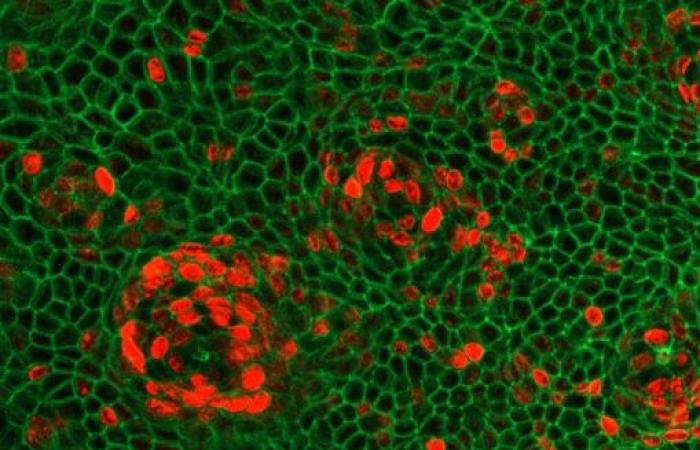
Inflammatory bowel diseases (IBD) include Crohn’s disease and acute ulcerative colitis. They are characterized by inflammation of the lining of the digestive tract linked to deregulation of the intestinal immune system. These diseases affect more than 250,000 patients in France.
Type III interferon: An obstacle to repair of the intestinal mucosa
Until now, most treatments have focused on reducing inflammation. Although biological therapies and anti-inflammatory medications have significantly improved the symptoms of IBD patients, only 50% achieve complete remission. Others often suffer from incomplete healing of the intestinal mucosa and frequent relapses.
In an article published in the journal thel, scientists have discovered a key mechanism that slows tissue repair in the intestines during inflammation. This discovery paves the way for designing treatments that could help these patients better repair their intestines and achieve complete remission.
Scientists have identified that a protein called interferon lambda or type III interferon (IFN-λ) plays a crucial role in delaying intestinal healing. Normally, IFN-λ helps fight viruses by preventing their replication, but in people with IBD, this protein is produced in abnormal amounts during inflammation. In the chronically inflamed intestines of these patients, IFN-λ can block the repair process, thereby delaying remission.
A key mechanism identified.
Using different transgenic mouse models, but also intestinal organoids (“mini-intestines” cultured from patient cells in a culture dish), scientists were able to trace the molecular pathway activated by IFN-λ. They found that IFN-λ is abnormally elevated in IBD patients, which leads to increased production of another protein, ZBP1, in intestinal cells. ZBP1 activates a cascade that destroys intestinal stem cells, preventing them from regenerating the epithelial layer.
This mechanism emerged during evolution to kill virus-infected cells, but during the dysregulated inflammatory responses typical of IBD, it can be activated even in the absence of viral infection. Indeed, scientists have demonstrated that under these conditions, the signal which activates ZBP1 and induces cell death comes from the inflammatory state of the patients.
Above all, using organoid models that distinguish between the acute inflammation phase and the repair phase, they realized that this mechanism which is active during acute inflammation, remains active during the mucosal repair phase. This finding is particularly important in a therapeutic context, as current therapies can calm inflammation but often do not result in complete recovery.
These discoveries open the way to a new therapeutic approach: by blocking type III interferons, it may be possible to restore the intestinal barrier, thus improving patients’ quality of life and reducing the risk of serious complications.