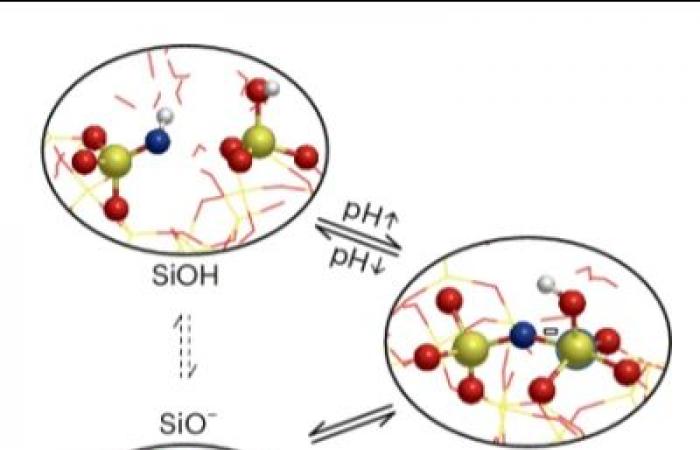
Silica (SiO₂) is the essential raw material for glass manufacturing. It is also used to produce ceramics and is used in the composition of concrete and cement. An insulating material, it is now found in transistors, chips or sensors, cosmetics or drug delivery systems. Frequently in contact with water, the presence of moisture on the surface can modify its local structure and influence its properties, or even alter its performance.
To progress in understanding water/oxide surface interactions, scientists from the Analysis, Modeling and Materials Laboratory for Biology and the Environment (CNRS/Université d’Evry Paris-Saclay) carried out a study combining experimental spectroscopy and modeling. theoretical. They reveal a complex chemistry that has been ignored until now due to the lack of spectroscopic experiments on aqueous interfaces capable of directly probing the surface properties of oxides.
For this, they developed a specific nonlinear optical experiment of the SFG (Sum Frequency Generation) type.
. They thus managed to probe for the first time directly the surface oxide in contact with liquid water while the usual experiments probe water in contact with the surface. The scientists then coupled these measurements with ab initio DFT-MD molecular dynamics simulations which take into account the overall state of the surface, namely the presence of Si-OH entities and strong (covalent) Si-O bonds. -If. They thus access the chemical reactivity of surface Si-O-Si bonds as a function of pH.
Finally, using a new method called ‘pop-model’, they computationally reproduced the SFG nonlinear optical spectroscopy results in a pH range from 2 to 12. These results allowed them to correlate chemical reactivity of the bonds Surface Si-O-Si to the acidity of the environment, but also to identify some criteria to rationalize this reactivity, in particular the local geometry of the surface sites.
Work published in the journal Nature Chemistry which open up perspectives in the fine characterization of the chemical and/or physical reconstruction of surfaces of numerous oxides in contact with liquid water.
Editor: CCdM