VIENNA – Fecal microbiota transplantation is indicated in cases of relapse of colitis Clostridium difficile. However, a non-inferiority study presented at the European Congress of Gastroenterology (UEG Week 2024) by the According to Frederik Emil Juul (Clinical Effectiveness Research Group, Oslo University Hospital, Norway), suggests not only that it would be as effective as first-line treatment as vancomycin, but that the relapse rate would also be lower. The question is on the table.[1]
Fecal microbiota transplantation to achieve lasting clinical cure
Antibiotic-associated colitis due to infection with Clostridioides difficile (formerly Clostridium difficile) represents a growing health problem worldwide. The recommended treatment for a first infection is currently the administration of antibiotics, notably vancomycin, the recommended treatment, or fidaxomicin [2]. Antibiotic treatment of infections Clostridioides difficile allows a cure rate of 71 to 79% [3]. However, recurrences are common.
Fecal microbiota transplantation (FMT) is indicated for recurrent infections with C. difficilebut its benefits in cases of primary infection are little known. Norwegian researchers thus designed a study whose objectives were to evaluate whether the transplantation of fecal microbiota was not inferior to standard antibiotic treatment in obtaining a lasting clinical cure of primary infection with C. difficile.
A demonstration of the safety and non-inferiority of FMT
This multicenter randomized non-inferiority trial conducted in Norway demonstrates that fecal microbiota transplantation is safe and non-inferior to vancomycin for the treatment of primary infection with C. difficile. For this demonstration, adult patients with acute infection with C. difficile (≥ 3 loose stools per day and toxin test C. difficile positive) without a history of infection with the bacteria in the previous year were randomized to receive either rectal fecal microbiota transplantation or oral vancomycin (125 mg four times a day for 10 days). Of the 104 randomized patients, 100 received fecal microbiota transplantation or the first dose of vancomycin and were eligible for analyses.
Each fecal microbiota transplant was derived from 50 g of stool from healthy donors aged 16–30 years, prepared at the University Hospital of North Norway (Harstad, Norway). The primary endpoint was the percentage of patients achieving clinical cure (firm stools or ≤ 3 stools per day) after 14 days without recurrence in the following 60 days, and the absence of additional treatment, according to an intention-to-treat analysis. modified.
Secondary endpoints relate to adverse events occurring during treatment. The study was not blinded to the clinicians who treated the patients.
Similar rates of clinical cure without recurrence
1,219 patients were selected. Among them, 51 (4.2%) received FMT and 49 (4%) were treated with vancomycin. The median age was 70 years (51-79) for the FMT group and 71 years (58-77) for the vancomycin group.
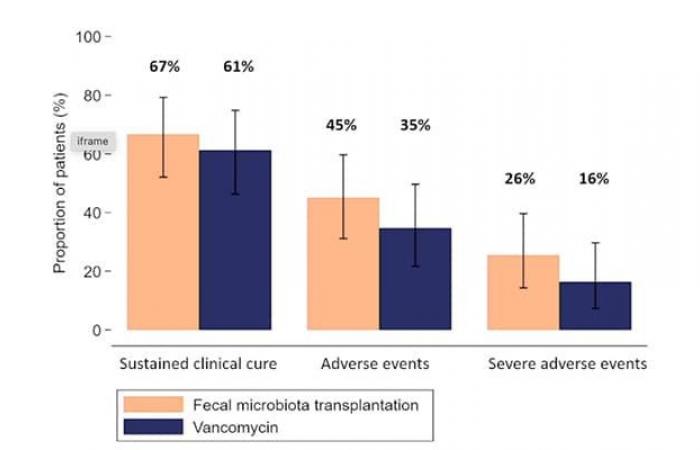
Clinical cure without recurrence was observed in 34 of 51 patients (66.7%; 95% CI: 52.6 – 78.3%) who received FMT compared to 31 of 49 (63.3%; 95% CI: 48.9 – 75.6%) under vancomycin (difference in therapeutic success of 3.4%; 95% CI: -21.2 – 28.0%; p for non-inferiority of FMT/vancomycin: 0.001) .
Vancomycin versus fecal microbiota transplantation in the treatment of primary C. difficile infection (Slide presented at UEGW 24 by Professor Frederik Juul)[1]
Clinical healing at 14e day, without recurrence with or without additional treatment, was obtained in 40 out of 51 patients (78.4%; 95% CI: 64.9 – 87.7%) in the FMT group, compared to 31 out of 49 (63.3 %; 95% CI: 48.9 – 75.6%) under vancomycin (difference in therapeutic success of 15.2%; 95% CI: -8.0 – 38.3%; p for non-inferiority:
No difference in adverse effects was observed between the two groups.
“According to our study, fecal microbiota transplantation is considered safe and not inferior to vancomycin in the treatment of primary infection with It’s difficult »concluded Professor Juul. An interesting avenue for antibiotic-saving purposes.
Links of interest from experts: FE Juul addressed the subject of fecal microbiota transplantation, free of charge, during a scientific meeting organized by Tillotts Pharma.
Register for newsletters de Medscape : select your choices